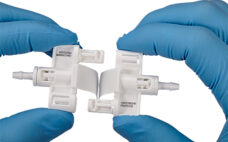
Small-volume biopharmaceutical processes continue to grow as more biopharmaceutical, cell therapy and gene therapy companies develop products. A 2021 Association for Regenerative Medicine (ARM) report states that there are 1,085 cell, gene and tissue-based therapy developers worldwide. These companies are engaged in many small-volume (<10L) processes including early-stage drug development and autologous therapies. The increase in small-volume processes coincides with the ongoing need to create fast, reliable aseptic closed systems. Historically, few convenient options have existed to facilitate sterile processing.…
Sponsored Content
Rapid Process Development: How a Synthetic Depth Filter Offers a Platform Approach for Efficient Virus Filtration Operations
Virus filtration is a critical unit operation in recombinant protein purification processes. Process development of virus filtration operations typically strives to maximize filter capacity and improve process robustness. Adsorptive prefilters are often used to remove higher order protein aggregates and improve filter capacity. While adsorptive membrane prefilters offer easy scale up and implementation, their narrow operating window may result in extensive process development screening studies. By contrast, synthetic depth filters offer a wide operating window and the potential for a…
Drug Discovery: Screening Approaches for Rapid Assessment of Target Tractability
This webcast features: Nuska Tschammer, Head of DEL Lab Operations, WuXi AppTec. In recent years, increasing use of genetic, transcriptional and knock-out technologies led to numerous biologically validated targets, many of them in the category “first-in-class”. The core purpose of experimentally based tractability assessments is to evaluate if the target of interest can be modulated by a chemical entity. In the past, extensive HTS screening efforts were initiated to find starting points for the small molecule drug discovery. Today, direct…
Not All cGMP Transfection Reagents Are Made Equal: Pharmaceutical Versus Medical Device cGMP Manufacturing
cGMP grade transfection reagents should be produced and formulated following strict guidelines. Some suppliers manufacture transfection reagents according to medical devices cGMP standards which makes the reagents suitable for viral vector manufacturing in the United States but not suitable for direct administration into humans. Those are often labelled as “for research use only and further manufacturing. Not for use in humans or animals”. Others comply to pharmaceuticals cGMP guidelines, making the transfection reagent suitable for viral vector manufacturing as well…
Developing Methods for Comparability Studies of Therapeutic Monoclonal Antibodies: Minimize Time, Maintain Quality
This webcast features: Kalhari Silva, PhD, Head of Scientific Research, Custom Biologics, and Bob Dass, PhD, Senior Scientist, Octet Applications, Sartorius. Quantifying critical quality attributes accurately and precisely is an important aspect of regulatory compliance. There is greater demand to optimize processes by integrating advanced analytical tools that maximize quality, safety, and efficacy of biotherapeutics. Kalhari Silva, from Custom Biologics, will provide insight on how her team designs and establishes methods suitable for comparability studies that allow for their future…
Lab Scale Depth Filtration with FILTROSPIN™ 20
FILTROSPIN™ 20 by FILTROX enables you to quickly and efficiently test different depth filters or quickly purify a small amount of liquid. The filters are inserted into standard 50 mL centrifuge tubes (Labcon® SuperClear® 50 mL tubes). The liquid is then filtered using a centrifuge or vacuum. In order to evaluate the most suitable and optimized production process, small-scale trials are the key factor to start with. With the help of laboratory scale trials, errors and other issues can be…
Application of Dry Granulation to Facilitate Raw Material Handling
Pharmaceutical and biopharmaceutical manufacturing relies on the timely and seamless orchestration of many steps to meet aggressive timelines and ensure operational efficiency. A fundamental element of the processing workflow that can impact schedules is the use of multi-ton quantities of bulk raw materials such as buffers, salts and stabilizing chemicals. These raw materials are typically prepared in a just-in-time manner to meet dynamic production needs and enable rapid changeovers.
Optimizing Feed Strategies with Cellvento® ModiFeed Prime Comp: A Scalable Process
Development of upstream processes, that maximize productivity without increasing complexity, relies on the right combination of medium and feed and the ability to maintain tight control of culture conditions. In fed-batch mode, cells are initially grown in a relatively lean production medium followed by regular feed supplementation to achieve the desired product yield. This App Note presents an optimized feeding strategy and demonstrates how a new, easy-to-hydrate, single-part, pH neutral feed can support optimal cell growth and high productivity of…
Intensifying Upstream Processing and the Implications for Media Management
Process intensification offers many benefits but implementing PI can introduce an unexpected challenge of managing larger media volumes. This whitepaper covers the implications of media management when developing PI strategies. Key Learning Objectives: Gain the tools and knowledge needed to confidently assess intensification options with a focus on media management for new or existing facilities. Follow the media journey from prep to use, exploring potential logistical pitfalls in the management of increased media volumes associated with process intensification. See how…
Realizing the Potential of Immune Cell Therapies
Remarkable progress is being made in the treatment of cancer. Among the most promising approaches is immune cell therapy, the most prominent type of which is CAR-T therapy. CAR-T therapies have been approved since 2017 and hundreds of clinical trials are now underway. While the race to develop these complex therapies continues to pick up speed, strategies to reduce the steep costs associated with their manufacturing are evolving at a rapid pace. In this article, we outline the factors contributing…