Recombinant viruses (e.g. lentivirus and adeno-associated-virus) can be used as human gene therapy vectors. They are mainly produced in adherent cell cultures (e.g. HEK293T, A549, VERO) in Roller Bottles or multiple-tray-stacks using either transient transfection or infection strategies. Therefore, the Integrity® iCELLis® (ATMI LifeSciences) line of bioreactors offers a new production alternative with stronger process controls and ease of scale-up. The iCELLis bioreactor is designed for adherent cell culture applications. Cells grow on microfibers carriers packed in a fixed-bed…
Sponsored Content
Is the Gold Standard Tarnished? Weaknesses in the Standard Method Compromise Your RMM Validation
Introduction of a new Rapid Microbial Method (RMM) requires a full validation. The purpose of the validation is to prove that equivalent or better results are obtained using the new method when compared to the compendia. One assumes that the result obtained with the compendial method is absolute and is the “Gold Standard” by which to compare the new test. In reality, the compendial method exhibits a number of weaknesses that compromise the integrity of the validation. The standard requires…
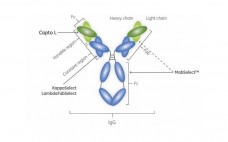
A Platform Approach for the Purification of Domain Antibodies (Dabs)
A three-step purification process for a Dab successfully developed and verified. The step yield ranged between 86% and 99%, giving a total process yield of approximately 81%. The ECP in the start sample was more than 200 000 ppm whereas the final sample contained only 5.5 ppm. The endotoxin content in the feed was approximately 2 million endotoxin units/mg of protein and the final sample was below the limit of quantification. Protein L leakage was undetectable in all samples. This…
MFI for Particle Characterization of Biopharmaceuticals Today
Micro-flow imaging (MFI) is a sensitive, simple and automated method for the analysis of sub-visible particles and translucent protein aggregates that provides particle size, count and morphology. Because it quantifies particle shape, this technology can discriminate groups of particles from each other, and evaluate how these groups change over time. In this article, we provide an overview of how biopharma is using MFI to characterize and monitor their protein formulations in new and emerging applications.
POROS® CaptureSelect™ Affinity Columns for Rapid, Small-Scale Purification and Sample Preparation of Recombinant Proteins
This application note describes a model system in which Enbrel® protein, a fusion protein consisting of TNF receptor and IgG1 Fc, is purified from a dilute sample matrix for further analytical characterization. The resulting purified protein was subjected to N-linked glycan structural analysis by mass spectrometry (MS) and its aggregation state was assessed by analytical ultracentrifugation (AUC). This data set serves as a model to demonstrate the capability of the POROS® CaptureSelect™ product line for the affinity purification of biomolecules…
Mirus TransIT-PRO® Transfection Kit for Biotherapeutic Protein Production
Decrease time to produce usable protein by maximizing target protein yields through transient transfection. The TransIT-PRO® Transfection Kit uses animal origin free components designed for high and reproducible nucleic acid delivery into suspension CHO and 293 derived cells. Since it is compatible with varied media formulations, the same media can be used for both transient and stable expression. The TransIT-PRO outperforms linear PEI in protein yield, while providing a cost-effective alternative to FreeStyle™ MAX and 293Fectin™ Transfection Reagents.
Control Over Your Microbial Identification Using the Gold-Standard Genotypic Method
Eliminate your outsourcing needs! Gain visibility and control. Learn how the next-generation, high-throughput MicroSEQ® system delivers right-first-time accuracy and give you complete control over microbial identification, by owning the system, the process and data analysis.
Charge Heterogeneity Analysis in 10 Minutes
Platform methods, high resolution and ease of use have made the iCE system the gold standard for protein charge heterogeneity characterization for biopharmaceuticals. At 15-18 minutes per sample, iCE methods are already fast and simple but now they are even better. In this white paper we describe a high throughput 10 minute charge heterogeneity analysis method and performance improvements that provide automated analysis of 100 samples at a time.
How to Achieve Greater Efficiency in Biopharmaceutical Process Development
Read about GE Healthcare Life Sciences’ support from efficient process development to production through products, consulting services as well as specific, individual collaborations. “Our aim is to help bioprocessing teams to map the optimal plan to transform an idea into results, with greater flexibility and confidence, reducing time and costs along the way. Our global team of bioprocessing experts will support you in the optimization and troubleshooting of existing unit operations or in the design of efficient and cost-effective processes…
Cookie Cutter Proteolysis: Achieving Reproducible, Efficient Digestions for Proteomic Workflows
Cookie Cutter Proteolysis: Achieving Reproducible, Efficient Digestions for Proteomic Workflows  Protein sample preparation workflows for mass spectrometric analysis that involve proteolysis are often labor-intensive, time consuming and user dependent. These workflows often involve digestion, solid phase extraction, drying, and re-suspension prior to reversed phase separation into the mass spectrometer. The introduction of variability at many of these steps hinders discovery initiatives as well as the ability to convert these discoveries into viable assays.  Recently, an automated protein digestion…