Monoclonal antibodies (MAbs) are very successful for treatment of several different cancers and tumors. However, the low tissue penetration has led to the development of smaller sized biopharmaceuticals (Ab fragments) such as Fabs, single chain Fv (scFv) and domain antibodies (Dabs). These molecules lack the Fc part of the antibody making a platform purification approach using Protein A impossible. However, with the introduction of the Protein L based affinity chromatography media (Capto™ L) new possibilities are introduced for capture of…
Sponsored Content
Continuous Bioprocessing: A CMO’s Perspective
The maturation of many process industries is marked by transition from batch to continuous processing. Often this move to con-tinuous processing determines the winners in the industry. Will the bioprocess industry go the same way? The analogy is often drawn to the chemical industry where continuous processing is widely applied. Although available for many decades continuous bioprocessing is not yet a mainstay of the industry. Currently there is a ground swell of feeling within the bioprocess industry that the time…
Enhancing Efficiency and Economics in Process Development and Quality Control of Biotherapeutics
Analytical techniques that measure protein quantity and quality are used in nearly all stages of research, process development, quality control and manufacturing of biotherapeutics. UV spectroscopy, ELISA and HPLC have been in use for decades for protein quantitation in physiological and process samples, and continue to be the workhorses despite their many limitations. Biopharmaceutical companies have enthusiastically adopted Pall ForteBio’s Octet® systems due to their high throughput capabilities, decreased sample preparation requirements, and low cost of operations. The Octet systems…
Anticipating Success: Meeting the Inherent Challenges of Complex Drug Substances
Realizing the full potential of a novel injectable drug compound is no small task. In the months and years that lead from the exciting discovery phase to the rigorous demands of a commercial launch, unexpected scientific and technical challenges can slow development to a halt, often at key stages. A careful, systematic approach to identifying where and why these roadblocks can occur is fundamental to staying on course. Just as important is a robust, repeatable process design focused on retaining…
Designing Quality into the Product: Early Developability Assessment in Biopharmaceutical Development
The ultimate objective in the development of any new therapeutic candidate is the validation of its mechanism of action and therapeutic efficacy in a clinical setting. Three important areas of product development critical to determining the success of a new drug candidate are: manufacturing, safety, and delivery of the product to patients. Early attrition observed during preclinical stages can often extend development timelines or require additional process optimization, therefore costing the developer more time and money. It is desirable to…
Validation of the New Single-Use Freeze-Pak™STS Storage and Transport Solution Containers
Production of biotherapeutics whether for clinical development or large scale manufacturing campaigns intended to be converted to final drug product often involves frozen storage. Frozen storage provides manufacturing process flexibility while enabling long-term product stability. Products are frozen and stored using a variety of technologies including stainless steel vessels, bottles, carboys and single-use bags. Use of bags has become popular due to their low investment cost and process flexibility attributes. Single-use bags intended for freezing and storage are often made with films using EVA and/or LDPE with product routinely blast frozen and stored to -30°C in a cold storage warehouse. During freezing and transport, the bags will typically experience temperatures well below -30°C ranging from -50 to -80°C. Under these conditions, the bags have to endure a wide variety of stresses (film brittleness, volume expansion, etc.) impacting integrity. With applications (like working cell banks for example) requiring lower temperatures for maintenance of long-term product stability, frozen storage films and containers designed for these conditions are needed. The new single-use Freeze-Pak™STS (FP-STS) frozen storage and transport solution containers from Charter Medical, Ltd. are manufactured using a unique polyolefin monolayer film designed for freezing applications The FP-STS bags (including tubing and connectors) have been validated for storage to -80°C, while the Freeze-Pak™ film remains flexible to temperatures as low as -196°C. The new Freeze-Pak™ STS bags deliver the flexibility and durability required for frozen storage and transport.
E. coli Cultivation in a 12L and 120L CELL-tainer™ Single-Use Bioreactor
Single-use bioreactors are usually applied in the biopharmaceutical industry for mammalian cell culture processes. For microbial processes, concepts like the CELL-tainer® technology allow comparable gas-liquid mass transfer rates like in stirred tank reactors. In the CELL-tainer, the rocking motion of the bag is generated with a combination of a vertical and horizontal movement. Due to a 2-D rocking motion, the turbulence in the liquid is intensified. Thus, volumetric oxygen transfer rates (kLa) of over 400 h-1 could be achieved. Recently, the successful scale-up of an Escherichia coli nutrient-limited fed-batch cultivation from the 15 L to the 150 L scale in the CELL-tainer single-use bioreactor has been conducted. A final biomass concentration of 45 gL-1 within 24 hrs was obtained in cultivations proving the general suitability of this reactor concept for the application of bacterial processes. The combination of intelligent software sensor control strategies and currently improving (single-use) sensors will lead to a reduction of current drawbacks and improve control of bacterial fed-batch processes. The availability of single-use bioreactors for microbial cultivations widens their potential, not only in biopharmaceutical processing, but also as a pre-culture bioreactor for large scale processes and as a suitable tool in bioprocess development.
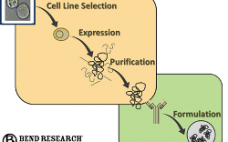
Driving Optimal Cell-Level “Observability” and Data-Driven Process “Guidance”
In the spirit of implementing PAT (Process Analytical Technology), biopharmaceutical companies are striving to gain a more fundamental understanding of what is happening to the cells within their bioreactors. Implementation of online tools like Raman and Dielectric Spectroscopy are helping to provide insight, yet there is still a gap integrating this data with off-line measurements such as cell density, viability, metabolite levels and titer. Bend Research, in collaboration with major biopharmaceutical companies, is working to advance MAST, a modular, automated sampling platform that can provide samples directly from bioreactors to analytical devices, while maintaining process sterility. In this webcast, Clint Pepper, Ph.D. and Lisa Graham Ph.D., P.E. of Bend Research focus on this overall cell-level “observability” concept and illustrate how the MAST platform is incorporated.
Selecting a Transfection Reagent for Large Scale Protein Production in Suspension 293 Cell Types
Decrease time to produce usable protein by maximizing target protein yields through transient transfection. The TransIT-PRO® Transfection Kit uses animal origin free components designed for high and reproducible nucleic acid delivery into suspension CHO and 293 derived cells. Since it is compatible with varied media formulations, the same media can be used for both transient and stable expression. The TransIT-PRO outperforms linear PEI in protein yield, while providing a cost-effective alternative to FreeStyle™ MAX and 293Fectin™ Transfection Reagents.
Bioreactor Monitoring – Best Practices for Cell Counting, Sizing, and Viability
Manufacturers utilizing bioprocesses for production need accurate and reliable tools for monitoring bioreactors. The key parameter that many bioprocess engineers rely on is viable cell density. This is a compound parameter arising from knowledge of the cellular concentration and the total cellular viability. Viable cell density is the mathematical product of these two primary pieces of information. In order to minimize variability, the detection technique of each of the primary measurements (concentration and viability) must be understood separately.
In this educational webcast, Matthew N Rhyner, PhD., Global Analytical Marketing Manager at Beckman Coulter Inc, discusses two of the most powerful techniques for assessing concentration and viability: the Coulter Principle and flow-based imaging. Join Dr. Rhyner as he compares the strengths and weaknesses of these techniques and discusses competing technologies. The webcast concludes with some recommendations for best practices in monitoring cell count, size, and viability.