Unlike chemically synthesized drugs, whose structure is known and reproducible, biological drugs are derived from living cells and are sensitive, complex mixtures requiring cutting-edge biological technologies for their production. The growing importance of biosimilars in recent years is reflected in a corresponding rise in market value. The value of the global biologic therapeutic drug market reached approximately US$230 billion in 2014 and, according to BCC Research, will increase to nearly $390 billion by the end of 2019. This corresponds to…
Sponsored Content
Continuous Chromatography Is Now Possible for Clinical Manufacturing
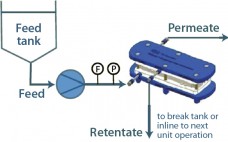
Intensified and integrated bioprocess technologies are creating a paradigm shift toward more efficient, higher flexibility facilities for biopharmaceutical manufacturing. Continuous technologies that are designed as single-use systems help to greatly facilitate process intensification, delivering further efficiencies with reduced set-up times and elimination of the need for cleaning and cleaning validation. Chromatography is often considered to be a challenging bioprocess step, which has caused great interest in a simplified, safer solution. Continuous multicolumn chromatography using a single-use flow path is an…
Development of a High-Performance, Integrated, and Disposable Clarification Solution for Continuous Bioprocessing
Current bioprocesses combine fed-batch cell culture with batch-wise downstream processing steps. To achieve integrated upstream and downstream continuous manufacturing, the industry has been in need of a continuous cell separation and clarification solution for bioprocess fluids from bioreactors. The Cadence Acoustic Separator from Pall Life Sciences provides this solution, with continuous first-stage clarification without the need for filter media in a scalable, single-use format with no negative impact on product attributes. The Cadence Acoustic Separator delivers cost and time savings…
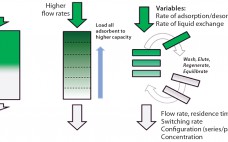
Optimizing Continuous Monoclonal Antibody Polishing By Using Coupled Unit Operations
The biopharmaceutical industry is under a great deal of pressure to modernize manufacturing to meet the challenges of production at vastly different scales for niche drugs as well as for expected massive blockbusters, biosimilars, and regional manufacturing. To address these challenges, the biopharmaceutical industry is embracing process intensification through single-use and continuous processing technologies. Implementing these technologies offers increased productivity and manufacturing flexibility and reduces the footprint, capital outlay, and operating costs. Pall Life Sciences has developed several technologies designed…
Enabling Continuous Processing Using a Step-by-Step Approach
Mario Philips is Vice President and General Manager of Single-Use Technologies at Pall Life Sciences. In February, he spoke with BPI publisher Brian Caine and editor in chief Anne Montgomery about Pall’s commitment to enabling continuous processing and its development of single-use technologies. In that discussion, he addressed some major process bottlenecks and Pall’s solution to them, including centrifuge replacements by continuous acoustic wave separation, continuous chromatography with multicolumn chromatography technology platform, and a simplified version of tangential-flow filtration. Read…
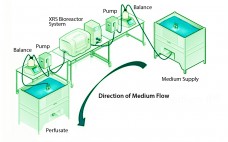
A Look At Perfusion: The Upstream Continuous Process
Although implementation of continuous manufacturing for biopharmaceuticals is in the early stages, continuous cell culture technology has been around for close to thirty years. Perfusion was initially developed in the late 1980s as a means for increasing protein titers (1). However, high costs driven by media consumption limited widespread commercial adoption. In the same time frame, advances in cell line engineering, media composition, and bioreactor design led to 10-fold increases in titers for batch and fed-batch modes, eliminating the first…
Flexible Automation for Continuous Unit Operations
Continuous processing has the potential to provide significant cost and time savings for biopharmaceutical manufacturing, but that potential can be realized only if appropriate automation solutions are available for continuous flow between disparate upstream and downstream operations. Pall Life Sciences’ Allegro MVP system, a fully automated bioprocessing system designed for use in upstream and downstream single-use processing, enables flexible automation and thus facilitates continuous biopharmaceutical manufacturing. This article presents the results obtained using the Allegro MVP system in combination with…
A comparative study of MiniTEMâ„¢ versus DLS Zetasizer Nano and NTA NanoSight NS300 performance when analyzing < 50 nm particles
Characterization and analysis of subvisible particles of biological origin can be challenging or give insufficient information. In this study Adeno associated virus (AAV) particles contaminated by host cell proteasomes are analysed with direct versus indirect methods performed in a standard laboratory setting to reveal the difference in performance and quality of the information obtained. The focus is on the ability to detect the AAV particles of interest and distinguish them from the contaminating proteasomes. The compact, tabletop MiniTEM system enables…
Modification of Glycans in Bioprocessing
This webcast features: Ryan Boniface, R&D Scientist, Thermo Fisher Scientific Protein quality is a topic of increasing importance as quality elements can have a significant impact on clinical behavior of a molecule. This talk will explore approaches to modify glycan patterns contribute to predicting, achieving and maintaining preferred glycosylation profiles. Glycosylation is a key product quality attribute for many biotherapeutic proteins expressed in CHO cells. N-linked glycans may display macro- and micro-heterogeneity; the degree of this variation can depend on…
Bioprocess Insights Webinars
The biopharmaceutical industry has developed into a multi-billion dollar market in just 30 years. Making decisions and setting up strategies in a rapidly changing environment can be challenging. The difference between a good and a great decision can have extensive implications for the future of a company. In this environment, knowledge and experience are what make the difference. Good insights about, for example, technical advancements, process economy implications, and sustainability aspects can help improve the individual decisions and strengthen the…