Levitronix® has released the first ultrasonic, single-use (SU) flowmeter on the market. Characterized by high accuracy and superior performance, LEVIFLOW® SU noninvasive flowmeters enable a highly precise and low-cost flow measurement. With an accuracy of <1% of reading and flow ranges from 1 mL/min to 80 L/min, they offer the highest level of accuracy for measuring liquids in biopharmaceutical and other applications that require FDA, USP-VI, BSE/TSE, and animal-component–free materials that can be gamma sterilized. Technical Background: Figure 1 illustrates the…
Sponsored Content
Buffer In-Line Dilution and LPLC: Analytical Accuracy with LEWA EcoPrime LPLC Systems
The new LEWA EcoPrime® LPLC chromatography and enhanced buffer dilution platform combines proprietary fluid design and the LEWA Intellidrive® pump technology to deliver unrivaled reproducibility and accuracy. The integrated buffer in-line dilution (BID) option prepares point-of-use buffers combining two unit operations into a single, space-saving skid. A single LEWA EcoPrime system has the flow range of up to two conventional LPLC units while delivering gradient accuracy of greater than ±1%. Because of its dynamic range, EcoPrime LPLC can bridge the gap…
Integrated and Scalable Continuous Chromatography: The Cadence BioSMB PD System from Pall
Bioprocess intensification leads to more efficient, flexible, and cost-effective biopharmaceutical manufacturing facilities. Single-use systems designed for continuous operation facilitate process intensification. Pall Life Science’s Cadence™ BioSMB PD platform is the first disposable flow path, continuous multicolumn chromatography solution that is fully scalable from a process development laboratory to good manufacturing practice (GMP) manufacturing. It provides an economically viable, disposable chromatographic solution for even the most demanding and costly chromatographic steps. During multicolumn countercurrent (also known as simulated moving bed, or…
Single-Use Systems: Balancing the Need Between Customization and Standardization
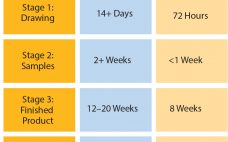
In June 2016, Parker domnick hunter began manufacturing in its new single-use facility in Birtley, Co Durham, UK. Here I discuss the approach taken in the design, testing, and manufacturing of single-use assemblies and some of the advantages they can have in terms of lead times, inventory control, and supply chain robustness. Challenges of Customization For some time, the message relayed to biopharmaceutical manufacturers was to design exactly what you want and need for each process. That meant a customized…
TOYOPEARL® AF-rProtein A HC-650F: Economic Advantages of Using a High Capacity Protein A Resin
TOYOPEARL AF-rProtein A HC-650F and two other commercially available protein A resins (one of them also a “high capacity” resin) were compared on a cost-per-gram of monoclonal antibody (mAb) produced basis at multiple resin prices from $7,000 to $17,000 per liter as well as three different column configurations. For in-depth comparison, the median resin price of $12,000 per liter was used as a basis to determine comparative production costs among the three resins tested. The configuration used to model what…
Efficient Removal of Endotoxins and Viruses By Anion-Exchange Chromatography
Various unit operations are needed in downstream processing (DSP) of biologics to remove process‑related impurities such as host‑cell proteins, nucleic acids, viruses, and endotoxins. This application note describes the potential of a salt‑tolerant anion‑exchange resin to achieve outstanding clearance of endotoxins and viruses. Introduction Endotoxins are lipopolysaccharides (LPS) originating from cell membranes of Gram‑negative bacteria. They are frequent contaminants of recombinant proteins produced in microbial expression systems. On the other hand, expression of more complex proteins in eukaryotic systems carries…
The Alcami Advantage
Alcami specializes in all phases of pharmaceutical development — from critical preformulation studies to commercial product life-cycle management. Over the past 30 years, Alcami has supported more than 500 investigational new drug (IND) filings and over 50 new drug applications (NDA), abbreviated new drug applications (ANDA), and new animal drug applications (NADA). Our fully integrated, comprehensive development services are well established and ready to help our clients get the most out of their portfolios. Principal Offerings
Contract Test Service
Associates of Cape Cod,® Inc. Contract Test Service (CTS) laboratory specializes in method development, bacterial endotoxin testing (BET), and glucan contamination. With the most extensive experience of any endotoxin testing laboratory in the world, CTS is GMP compliant, ISO registered, and licensed by the US Drug Enforcement Agency (DEA) as a laboratory capable of handling all controlled drug substances except those included in Schedule I. Endotoxin testing can be performed in accordance with US Food and Drug Administration (FDA), United…
Expanding CDMO Services to Meet All Your Manufacturing Needs: From Development to Commercialization
Avid Bioservices is a contract development and manufacturing organization (CDMO) specializing in mammalian cell culture process development and CGMP production of clinical and commercial-scale monoclonal antibodies, recombinant proteins, and enzymes. Committed to the success of our clients, our team constantly strives to build partnerships that extend well beyond the delivery of your product. By combining our knowledge and experience with a flexible and efficient approach, we can meet your specific project requirements Established CDMO with Proven Capabilities
Smart Biologics Development and Manufacturing, and SMARTag™ Technology: For ADCs, Bioconjugates, and ADVASEPT® Aseptic Filling
From drug and biologic development to delivery technologies and supply solutions, Catalent is the catalyst for your success. With 80+ years of experience, we have the deepest expertise, broadest offerings, and the most innovative technologies to help you get more molecules to market faster, enhance product performance, and provide superior, reliable manufacturing and improved results. Catalent is your strategic partner for biologic drug development and manufacturing success. SMARTag™ technology offers a new toolkit to optimize antibody–drug conjugates (ADCs) and bioconjugates.…