For over 75 years, Kerry has earned its reputation for reliability and excellence in serving the biotechnology, pharmaceutical, and nutrition markets. We deliver innovative solutions to assist customers increase cell proliferation, extend cell viability, and increase target protein production in biotechnological production systems. Every day, we expand our capabilities to meet the changing needs of the biotechnology market. Kerry’s products have evolved with market trends in cell culture over the past 50 years to fulfill our customers’ requirements and have…
Sponsored Content
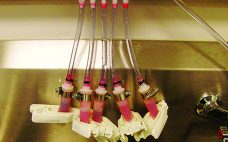
Working with Single-Use Industry Partners to Make a Better Tube Clamp
During the fall of 2014, we received requests from a couple of large, single-use technology (SUT) system integrators asking whether we could develop a new tube clamp for their SUT assemblies and skids. There were several motivating factors behind these requests. As bioreactor skids became larger and larger (with thousand-liter and larger SUT containers), technicians had increasing difficulties assembling current clamps onto tubing. In some cases, technicians were lying on their stomachs and reaching under the bioreactors trying to install…
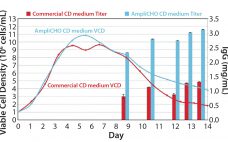
Simplify and Intensify Cell Culture: XCellâ„¢ ATF Systems for Cell Culture Process Intensification Now Available in Single-Use Format
A finalist for the 2012 BPI Technology of the Decade Award, XCellâ„¢ ATF systems consistently yield high cell retention, concentration, and viability, enabling efficient and productive perfusion and N – 1 perfusion as well as cell banking and seed-train cell culture operations. XCellâ„¢ ATF devices are now available in single-use format, in addition to stainless steel. The new single-use devices deliver 100% cell retention and laboratory-to-production scalability like their stainless steel counterparts but without the need for autoclave sterilization and lengthy…
Modern Peptone Manufacturing: Raising the Standard for Fermentation Substrates
Peptones from the Solabia Group represent the result of nearly 50 years of strategic activity and savoir-faire. As key components in industrial fermentation, they contribute to a range of products, from probiotics and vaccines to specific bacterial metabolites in cosmetics. Although they are often perceived as replaceable commodities with similar sounding names, that misconception can lead to significant problems. Peptones differ in sourcing as a function of a producer’s manufacturing experience, raw materials, and (most important) production site itself and…
Genderless Sterile Connectors: How They Can Increase Efficiency, Saving You Time and Money
Standardizing equipment in biopharmaceutical production streamlines operations, saving end users time and money. An area ripe for standardization is the use of genderless connections, those in which the connector halves are identical in design. Historically, single-use connections have involved male and female halves, but using two different parts can create several inefficiencies and complications, such as Increased ordering complexity Greater risk of specifying the wrong system or connection, resulting in downtime and last-minute adjustments Longer lead times Increased stocking requirements…
Increased Clarification Capacity: Using Filter Aid and FILTRODISCâ„¢ BIO SD for an Economical Filtration
Clarification of fermentation broths is one of the most important steps in bioprocessing. The first purification step after fermentation is the cell harvest, which is designed to remove cells and cell debris as well as to reach maximum product yield in compliance with existing regulatory environments. Standard technologies (centrifugation, separators, membrane, and depth filtration) can no longer handle the high particle loads (>108 cells/mL) in an economical way. Deciding on the right purification system involves addressing questions about process performance,…
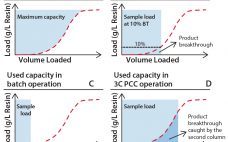
Use of Dynamic Control in Periodic Counter-Current Chromatography
Interest in continuous bioprocessing is growing rapidly, and such an approach has shown to have a potential in providing gains in both productivity and savings in cost of goods sold. The ÄKTAâ„¢ pcc 75 chromatography system can be used for purification of target proteins in continuous downstream processes using periodic counter-current chromatography (PCC). PCC enables greater use of chromatography resin capacity, allowing sample loading to much higher levels than what is possible in traditional batch chromatography by using safety factors…
Designing a MAb Purification Process: Based on Bio-Sequential Chromatography
In batch-mode chromatography, operating conditions do not allow full use of the total media capacity (1, 2). Bio-Sequential Chromatography (BioSC®) is an open-loop process for the separation of multicomponent solutions (3). The unique column of the batch mode is replaced by a series of smaller columns (from two to six). This design maximizes the use of the media without product loss along the process sequences. The BioSC® Lab automated system is based on the multicolumn chromatography process, designed and provided…
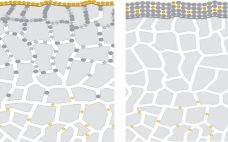
Adsorption Study of Polymer-Modified Cellufine CEX Resin
Cellulose is well known as a natural raw material that has mechanical strength, lower nonspecific adsorption, and good biocompatibility. In addition, cellulose particles have unique pore-size characteristics appropriate for chromatography of biopharmaceuticals. Ion-exchange chromatography (IEX) is an important step for biopharmaceutical manufacturing. Specifically, cation-exchange chromatography (CEX) can be used as a capture step in monoclonal antibody (MAb) purification. Recently, advanced IEX resins have been developed using polymer modification techniques. Initial screening of conditions such as pH and ionic strength is…
Platform Purification of Six Biosimilar Molecules Using Amsphere A3 Protein A Resin
Currently more than 70 biosimilar monoclonal antibodies (MAbs) are under development, and multiple originator MAbs are going off patent in the next three to four years. Protein A affinity media material remains the most important workhorse for the purification of monoclonal antibodies. Protein A media has a high impact on both development and manufacturing costs, particularly during early stage clinical phases. This note summarizes key performance parameters for JSR Life Science’s Amsphere A3 high-capacity protein A resin for six biosimilar…