A BPI Theater Roundtable at the 2016 BIO Convention On Wednesday, 8 June 2016, Gil Roth (president of the Pharma and Biopharma Outsourcing Association, PBOA) chaired a lunchtime roundtable titled, “Making the Correct Outsourcing Decisions.” He brought together a panel of four experts: Cory Lewis (vice president of business development and marketing at Cook Pharmica) Andrew Sanford (vice president of global business development for biologics at Catalent) David Powell (Pfizer CentreOne) Rajan Puri (senior director of business development at Therapure…
Sponsored Content
On-Demand Product Development Teams
A BPI Theater Roundtable at the 2016 BIO Convention On Wednesday, 8 June 2016, Joshua Speidel (managing director of the commercial practice team at the Latham Biopharm Group) chaired an afternoon roundtable titled, “On- Demand Product Development Teams.” He brought together a panel of four experts: Matt Rebold (director of business development at ICON Imaging and Technology — West Coast) Paul Jojorian (global head of technology transfer for Patheon Biologics) Eiry Wyn Roberts (vice president of research and development for…
CHOvolution™ Delivering Your Commercial Success: An Innovative Integrated Cell Line Development Platform
Celonic’s CHOvolution™ kit comes with a wide range of exceptional services and solutions, on top of high-performance cells. In addition to robust and highly efficient technology, we offer excellent technical support, including comprehensive protocols, audits, tailored workshops, and a 24-7 information access Internet-based platform with a hotline. For any drug developer or service provider, cell line development is one of the most challenging phases, especially when subsequent GMP compliance is required. To boost and support successful commercial introduction of biological…
Small Scale Escherichia Coli Cultivation Using BioBLU Single-Use and Reusable Vessels: A Comparative Study
In recent years, single-use bioreactors have gained more importance in animal and human cell culture. With its new line of BioBLU frigid-wall, stirred-tank, single-use vessels, Eppendorf offers premium solutions for microbial applications. In the following case study, reproducible process control was achieved with parallel-operated BioBLU 0.3f single-use and reusable glass vessels, both used in an Eppendorf DASbox® mini bioreactor system. Fermentation of Escherichia coli K12 led to very comparable results, thus proving tested single-use vessels to be an appropriate tool…
Continuous Processing and Integrated Facilities
Single-use technology has transformed the biomanufacturing industry, because single-use products provide significant savings over stainless steel infrastructure. Single-use technologies also create flexibility for biomanufacturers, enabling them to move from single-product processes to multiproduct facilities. As revolutionary and transformative as single-use technologies have been, the industry is on the cusp of another major shift, which comes with its own impressive benefits, but with some challenges as well. Continuous and Batch Processing Continuous processing has been used successfully for many years in…
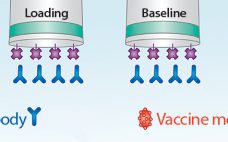
A Fast and High-Precision Influenza Vaccine Potency Assay
Fast and accurate determination of vaccine titer during manufacturing is important for understanding the performance of a development process and for scaling each process step. Although single radial immunodiffusion (SRID) has been the most commonly used technique for vaccine titer determination, it can be time-consuming and imprecise, requiring up to three days for results. The Pall ForteBio Octet® system offers a simple and direct method for measuring vaccine–antigen–antibody binding, capable of delivering high-precision analysis in as little as three hours.…
Application of DNA Looping: Efficient, Controlled, and Scalable Microbial Expression in pAVEway™ Expression Technology
Efficient expression of proteins is an integral part of the biopharmaceutical drug development process. With a range of expression systems, it is important to balance long-term manufacturing goals (e.g., cost of goods and productivity) while matching the expression system to suit the characteristics of a protein (e.g., product quality attributes and long-term stability). Finding the right expression system early on can make or break a therapeutic candidate as it moves from laboratory bench to clinical administration. FUJIFILM Diosynth Biotechnologies has…
Digital Communication in Algae Cultivation: Arc Technology Helps Optimize Yield and Growth Conditions of Algae
Today algae are commercially cultivated for pharmaceuticals, nutraceuticals, cosmetics, and aquaculture purposes. The abundance of vitamins, minerals, and trace elements as well as the fatty acid profile and thickening and stabilizing functions of their polysaccharides make algae valuable. Algae also can be used to produce biodiesel, bioethanol, and biomass that can be burned to generate heat and electricity. Like plants, algae use sunlight during photosynthesis. Photosynthesis is an important biochemical process in which plants, algae, and some bacteria convert the energy…
Boosting Profitability with Single-Use Powder Transfer
Many biopharmaceutical manufacturers are implementing single-use containment for transfer of media and buffer materials to prevent feedstock contamination and promote worker safety. But the type of containment system used can have a significant impact on productivity and profitability. Equipment should be evaluated and chosen carefully. Pertinent Questions Systems should be analyzed not only for their basic ability to contain powders of interest, but also for how efficiently they integrate into production process. For instance, could the system introduce productivity bottlenecks,…
Labfors 5 with LabCIP: Innovative Solution for High-Throughput Microbial Culture
The need for a rapid turn-around in quantity (and the loss of technicians in many laboratories) has created a trend toward implementation of single-use vessels. Their advantages include no time lost in cleaning or sterilization (especially for bench-scale, autoclavable systems), no chance of inhibition or cross-contamination due to poor cleaning, and no need for cleaning and sterilization facilities in a crowded laboratory. For production-scale steel bioreactors, fully automated clean-in-place (CIP) and sterilization-in-place (SIP) have been possible for decades. By contrast,…