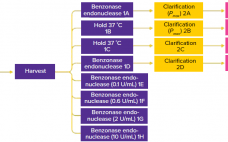
During production of vaccines and viral vectors, the size and quantity of extracellular nucleic acids must be reduced using endonuclease enzymes. Merck/MilliporeSigma’s proprietary Benzonase endonuclease is a genetically engineered nuclease derived from the Gram-negative bacteria Serratia marcescens. It attacks and degrades all forms of DNA and RNA. It is manufactured under good manufacturing practice (GMP) conditions and has a drug master file (MDF) in place with the US Food and Drug Administration (FDA), which can be cited in regulatory filings.…
September 2021
Single-Use Technology for Formulation and Filling: A Case Study from Swissfillon AG and Pall Corporation
Swissfillon AG is a contract manufacturing organization (CMO) based in Switzerland. Fully compliant with current good manufacturing practice (CGMP) regulations, it provides state-of-the-art aseptic filling for pharmaceutical and biotechnology companies, from clinical-phase materials to commercial quantities. This CMO specializes in high-value, difficult-to-fill products. Swissfillon recognized that adoption of single-use systems (SUS) on a commercial scale required major improvements in consistency and reliability compared to manual operations at pilot and clinical-trial scale. The single-use formulation and filling process, which includes an…
Ask the Expert: Facilitating Process Development Using a Microfluidic Perfusion Bioreactor
Suitable scale-down perfusion systems generally have been unavailable for process development (PD) activities. Some commercially available systems require daily media exchanges. No such system performs in a way that accurately represents large-scale perfusion, and none maintains sufficiently high cell densities. Kevin Lee (cofounder of Erbi Biosystems) joined BPI on 4 May 2021 to explain how his company’s Breez bioreactor system integrates all the functions of a stirred-tank reactor (STR) into a compact format that can facilitate PD, enabling one person…
Ask the Expert: Streamlining Mesenchymal Stem Cell Production — From Expansion Through Removal of Cryoprotectant
The number of cell therapy product candidates based on mesenchymal stem cells (MSCs) has grown steadily since their clinical debut in 1995. As of June 2020, clinical investigators were evaluating more than 1,100 such therapies. Scaling up MSC production remains challenging, however. On 31 May 2021, Hilary Sherman (senior scientist at Corning Life Sciences) presented an “Ask the Expert” webinar describing her company’s efforts to facilitate MSC workflows. Sherman’s Presentation Easing Expansion: MSCs have strong differentiation capability and can be…
Ask the Expert: A Novel Escherichia coli System for Controlled Release of Difficult-to-Secrete Proteins
Although Escherichia coli often enables dependable, highly productive expression of nonglycosylated recombinant proteins, the efficiency with which it secretes a target protein into culture supernatant can depend greatly on that molecule’s physicochemical properties. Some proteins remain trapped in periplasm, thus diminishing process yield and productivity. In June 2021, Marcel Thoen (head of Wacker Biotech’s Global Competence Center for Cell Line Development) described how his company’s improved ESETEC (E. coli secretion technology) solutions can address productivity challenges raised by difficult-to-secrete recombinant…
Ask the Expert: Critical Steps in Potency Assay Development
Biologics undergo extensive characterization to demonstrate their safety, purity, and efficacy. Jennifer Lawson (product manager for cell line, media, and testing solutions at Sartorius) highlighted the role of potency assays in that process. Because they reflect the complexity of biological systems, scientists must develop robust assays that will provide sufficient data for good-practice (GxP) applications. Lawson pointed out milestones in the bioassay life cycle and explored ways to help ensure method suitability. Lawson’s Presentation Development Criteria: Potency assay development requires…
Ask the Expert: HCP Analysis By Orthogonal Methods in Vaccine and Gene Therapy Development
Regulators require testing of drug products for process-related impurities throughout development to monitor product safety, purity, and efficacy. Low levels of most impurities can be inconsequential, but patient safety demands that host-cell proteins (HCPs) be eliminated or reduced to the lowest levels practical. Enzyme-linked immunosorbent assays (ELISAs) represent a key tool in that endeavor. Antibody-coverage analysis is one part of assessing a platform kit or custom HCP ELISA. In a 15 June 2021 webinar, Jared Isaac (senior scientist at Cygnus…
The Next COVID Challenge: Building an Arsenal of Vaccines
For months, international partners have pressured the United States about intellectual property (IP) concerns and access to SARS-CoV-2 vaccines. As the country emerges from the worst of the pandemic, President Joe Biden now has an opportunity to shift his administration’s focus to realizing its longstanding promise of making the United States “the arsenal of vaccines for the world” (1). However, the country cannot accomplish that goal just by exporting mRNA vaccines and waiving IP protections. The current generation of vaccines…
Kuhner TOM For Off-Gas Analysis in Shake Flasks
To facilitate and speed up process- and media development, online measurement techniques for shake flasks accompany manual sampling for maximum information output per cultivation. Off-gas analysis gives oxygen transfer rate (OTR), carbon dioxide transfer rate (CTR) and respiratory quotient (RQ) as quantitative measures of the physiological state of the culture. On shake flask scale, multiple cultivations are usually run in parallel. Off-gas analysis should therefore be cost effective, easy to handle and versatile to match various applications. Therefore, we developed…
Ask the Expert: Leveraging Quality Management Systems to Achieve Competitive Advantages
During a May 2021 “Ask the Expert” presentation, Jigisha Patel (vice president of global regulatory compliance and technical services at Spectrum Chemical Manufacturing Corporation) emphasized the need to minimize supply-chain contingencies that lead to variability across raw materials used in biopharmaceutical manufacturing. Deviations in raw-material specifications can jeopardize good manufacturing practice (GMP) compliance and reduce drug-product efficacy and stability. Patel explained how her company’s quality management system ensures that bioprocess materials will meet specifications and process needs. Patel’s Presentation Patel…