Servier will use 4D bioprinting technology to imitate human liver tissue in vitro, while STEMCELL Technologies is commercializing human pluripotent stem cell-derived kidney organoids. But can these models replace animal testing in preclinical research? French drugmaker Servier has (bio)inked a deal with Poietis to use its 4D bioprinting technology for the development and production of liver tissues. The tissue will be used to imitate human liver tissue in vitro in Servier’s preclinical testing. Poietis’ technology works through the “layer-by-layer additive…

Regulations
Sanofi and Regeneron gain approval for skin cancer MAb
The US FDA has approved skin cancer drug Libtayo, a programmed cell death protein-1 (PD-1) inhibitor developed through a Sanofi and Regeneron collaboration. In 2007, Sanofi teamed up with Regeneron Pharmaceuticals to discover, develop, and commercialize fully-human therapeutic antibodies. While the discovery collaboration ended last year, Sanofi still owns a 22% stake in Regeneron. The the firms also continue to develop and commercialize a number of monoclonal antibodies, including its metastatic cutaneous squamous cell carcinoma (CSCC) product cemiplimab which the two…
Catalent clears up FDA 483 concerns at Indiana plant
The plant in Bloomington received a Form 483 with five observations in May, but Catalent says a second US FDA inspection shows issues have been resolved. The US Food and Drug Administration (FDA) recently published a Form 483 it issued to Catalent’s biomanufacturing facility in Bloomington, Indiana following an inspection in April and May this year. The Agency made five observations at the 875,000ft2 production and fill/finish facility, which the contract development and manufacturing organization (CDMO) gained as part of…
Sanofi prepped for production after EU Nanobody success
Ten weeks after acquiring Ablynx, Sanofi has received European approval for lead Nanobody-based drug Cablivi (caplacizumab). In June this year, Sanofi acquired Belgian biotech Ablynx for €3.9 billion ($3.5 billion), adding the firm’s pipeline of biotherapeutics based on the small-sized antibody, or Nanobody, technology. Now the French biopharma firm is set to commercialize lead product caplacizumab (anti-vWF Nanobody) in Europe under the brand name Cablivi after the European Commission granted it marketing authorization for the treatment of rare blood-clotting disorder…
False biosimilar communications: Originators rebuff Pfizer’s claims
Pfizer has accused Amgen, J&J and Roche of sending misleading communications about biosimilars in a Citizen Petition asking the US FDA to issue guidance on such behavior. In a Citizen Petition published on August 22, Pfizer said communication tools intended to incentivize the adoption of and switching of biosimilars have led to a robust uptake of biosimilars in Europe, but in the US “payer reimbursement policies are in fact impeding adoption of biosimilars.” The firm continued: “Dissemination of false or misleading…
Novartis constructing $92m CAR-T plant as Kymriah arrives in EU
Up to 450 jobs will be created as Novartis commits to a production site for cell and gene therapies in Switzerland. The investment will support CAR-T drug Kymriah, which received European approval this week. A year after the US Food and Drug Admistration (FDA) approved Kymriah (tisagenlecleucel), the European Commission (EC) has granted the Novartis’ therapy approval to treat B-cell acute lymphoblastic leukemia (ALL). In the US, the chimeric antigen receptor T cell (CAR-T) product is manufactured at Novartis’ Morris…
Kyowa Hakko fermentation plant hit with FDA warning
Kyowa Hakko has received a US FDA warning letter citing GMP and data integrity issues at its amino acid production facility in Yamaguchi, Japan. The warning letter dated August 10 2018 came following an inspection of Kyowa Hakko Bio’s Hofu facility in September 2017 by the US Food and Drug Administration (FDA). Deviations from current good manufacturing practice (cGMP) for active pharmaceutical ingredients (API) cited by the Agency included failures in Kyowa Hakko’s quality unit, along with a lack of…
US made biosimilars: Competitive advantage or marketing spin?
Coherus says American healthcare providers and payers favor biosimilars made in the US over those made overseas. But an industry analyst has dismissed this saying location should not matter provided the facility is approved. Coherus’ second quarter 2018 contained several regulatory milestones relating to its candidate Udencya (pegfilgrastim-cbqv), a biosimilar candidate to Amgen’s Neulasta. A resubmission to the US Food and Drug Administration (FDA) was accepted in May, while in July Europe’s Committee for Medicinal Products for Human Use (CMPH)…
Jazz scales back Erwinaze push due to manufacturing disruptions
To minimize impact on patients, Jazz Pharmaceuticals has reduced promotion of its cancer drug due to continued manufacturing issues at its CMO Porton Biopharma Limited (PBL). Erwinaze (asparaginase Erwinia chrysanthemi), an asparaginase enzyme derived from the bacteria Erwinia chrysanthemi, is used for the treatment of patients with acute lymphoblastic leukemia (ALL) who have developed hypersensitivity to E. coli-derived asparaginase. The product is made for Jazz Pharmaceuticals by Wiltshire, UK-based and UK-government backed biomanufacturer PBL. However, the US Food and Drug…
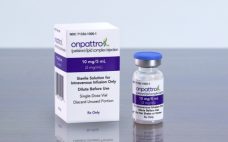
Alnylam’s success brings first RNAi therapeutic to US
The US Food and Drug Administration (FDA) has approved the first ever RNA interference (RNAi) therapy: Alnylam’s Onpattro (patisiran). Alnylam announced last week Onpattro has been approved in the US for the treatment of the polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. The product was hailed by the FDA as the first in a new class of drugs: small interfering ribonucleic acid (siRNA) treatments. “New technologies like RNA inhibitors, that alter the genetic drivers of a disease, have the…