This webcast features: Dmitrij Bugajev, R&D Scientist, and Jason D. Brown, Design Engineer, Thermo Fisher Scientific Single-use fermentors enable production facilities to utilize single-use technologies instead of traditional stainless-steel fermentor vessels, achieving equivalent expression with rapidly growing high-density cultures. In this webcast, we will compare some results from various processes with yeast and bacteria before and after technology transferred to single-use at our two facilities. We will give you our feedback as previous end users of stainless-steel fermentors and now…
Webinars
Webcast: Enabling Digital Chromatogram Review for a Faster and More Reliable Operation
This webcast features:Â Martin D. Jensen, Senior Engineer, FUJIFILM Diosynth Biotechnologies Chromatogram review is a monitoring method used to verify process performance in packed-bed chromatography processes. By observing key process parameters such as chromatography column outlet conductivity or UV absorbance, it is possible to identify the signs of a poorly packed column, resin degradation, or equipment malfunction. The industry standard practice relies on performing a paper-based qualitative visual comparison of chromatography profiles against a reference batch. This approach leads to a…
Strategic Development of Characterization and Quality Control Programs for Therapeutic Peptides
This webcast features: Ashleigh Wake, UK Business Development Director, Intertek Pharmaceutical Services Peptide therapeutics are a unique class of pharmaceuticals that may be regarded, in regulatory terms, as either conventional chemical molecules, biological entities or biosimilars. Slight changes in the structure, physicochemical properties, stability, and impurity profile of a peptide can provoke an adverse immune response; therefore, safety assessment is critical. Building a well-thought-out quality control (QC) strategy is key to meeting development milestones and complying with evolving regulatory requirements.…
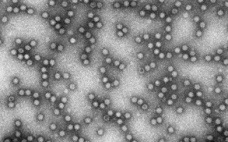
Predicting Viral Clearance Using a Non-Infectious MVM Surrogate in Downstream Development
This webcast features: David Cetlin, Senior Director, R&D, Cygnus Technologies As viruses can arise during the manufacture of biopharmaceuticals, regulatory agencies require viral clearance validation studies for each biopharmaceutical prior to approval. These studies are typically conducted in biosafety level (BSL)-2 facilities and require large capital and human resources. Due to these hurdles, process knowledge pertaining to viral clearance is limited during development and characterization. The use of an accurate, economical, and quantifiable non-infectious viral surrogate would enable downstream purification…
Application-Specific Enhancements to Thermo Fisher Scientific™ HyPerforma™ Single-Use Bioreactors
This webcast features: Ben Madsen, Engineer, Thermo Fisher Scientific The rapid growth of biotherapeutic manufacturing has created significant demand for workflow solutions featuring greater product yield, lower production costs, and accelerated development timelines. To address these demands, developers have moved away from “one-size-fits-all” approaches and are increasingly focused on solutions that address the specific needs of diverse bioproduction processes. Given this shift toward process-specific solutions, Thermo Fisher Scientific™ has introduced a series of application-specific enhancements to the HyPerforma™ Single Use…
To Compress or Not to Compress: What You Need to Know About Packing Resins at Process Scale
This webcast features: Dr. Mark A. Snyder, Manager, Process Chromatography, R&D Applications Group, Bio-Rad Laboratories Join our expert, Dr. Mark A. Snyder, to learn best practices and guidelines for successfully packing different types of chromatography resins. This includes incompressible resins like CHT Ceramic Hydroxyapatite Media as well as traditional compressible resins. CHT is an easy-to-use mixed-mode media and has high specific gravity, a rapid settling rate, and sensitivity to mechanical shear. These variations from traditional compressible resins have to be…
Cell Culture Media Manufacturing, Outsourcing and the Pandemic: An Essential Toolkit
This webcast features: Chad Schwartz, Senior Global Product Manager for Proprietary Cell Culture Media Formulations, and Eric Nalbach, Director of Product Management, Bioproduction, Cell Culture Media, Thermo Fisher Scientific The COVID-19 pandemic has had an unprecedented impact across the bioproduction industry, from supply chains to working practices. As organizations adapt to such changes, they have already experienced huge shifts in how the industry works. Those working in both research and manufacturing feel an obligation to employ all available resources to…
Accelerating mRNA-Based Therapy Development with Scalable Purification of In Vitro Transcribed mRNA
This webcast features: Kelly Flook, Senior Product Manager, Purification Products, Thermo Fisher Scientific The diversity of potential mRNA-based therapies has led to increased interest in using synthetic mRNA as a tool in the treatment of multiple diseases, such as cancer, stem cell therapies, and infectious diseases. Nevertheless, obtaining larger quantities of synthetic mRNA for clinical treatment remains a challenge. Currently available mRNA purification methods are becoming a bottleneck for large-scale manufacture as the limits of research-scale purification techniques are realized.…
CHOgro® High Yield Expression System: Achieve Higher Titers Faster in Suspension CHO Cells
This webcast features: Leisha Kopp, Applications Scientist, Mirus Bio Suspension Chinese Hamster Ovary (CHO) cells are often utilized for protein expression in biomanufacturing given their capacity for high-density growth in chemically defined media, human-biosimilar posttranslational modifications, and strong history of regulatory approval by the US Food and Drug Administration (FDA). However, many early stage researchers rely on HEK 293 systems for screening candidate compounds because of additional costs and time associated with production in CHO cells. The CHOgro® High Yield…
Enabling Large-Scale Production of Viral Vectors in the Gibco™ CTS LV-MAX Lentiviral Production System
This webcast features: Jonathan Zmuda, PhD, Director, Cell Biology, Life Science Solutions Group, Thermo Fisher Scientific The Gibco™ Cell Therapy Systems (CTS™) LV-MAX™ Lentiviral Production System enables scalable, high-titer production of lentiviral vectors using HEK293F-derived Gibco™ CTS™ Viral Production Cells adapted for high-density suspension growth in chemically defined, serum-free, and protein-free Gibco™ CTS™ LV-MAX™ Production Medium. Enhanced lentiviral production is enabled through the synergistic interplay of the LV-MAX components (including cells, production medium, supplement, enhancer, and transfection reagent), all of…