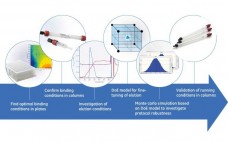
GE Healthcare Life Sciences MAb purification toolbox employs protein A chromatography media (resins) such as MabSelect SuRe™ or MabSelect SuRe LX, in the capture step. After the initial protein A step, there is a range of options for intermediate and polishing purification steps. One option is Capto MMC ImpRes, a new chromatographic medium based on a multimodal cation exchange ligand. This work describes a rapid procedure to establish a robust second step in bind/elute mode for the purification of a…
BPI White Papers
A Short History of Cell Culture Media and the Use of Insulin
A surprising history of cell culture media and the use of insulin, outlining the basic developments behind growing mammalian cells.
It will take you on a journey from the late 1800 where organ tissues were kept in balanced salt solutions -BSS- and later PBS, until the early 50’s synthetic media, over chick embryo extract and Eagle’s Minimal Essential Medium (MEM) or its modification by Dulbecco (DMEM). Finally describing insulin mimicking growth factors.
Gram Scale Antibody Production Using CHO Cell Transient Gene Expression (TGE) via Flow Electroporation
MaxCyte flow electroporation provides a universal means of fully scalable, highly efficient CHO-based TGE for the rapid production of gram to multi-gram level s of antibodies without the need for specialized reagents, expression vectors, or engineered CHO cell lines. In this technical note, we present data demonstrating the reproducibility, scalability, and antibody production capabilities of MaxCyte electroporation. Secreted antibody titers routinely exceed 400 mg/L and can exceed 1gram/L following optimization, thereby enabling multi-gram antibody production from a single, CHO cell transfection. In addition, we present data showing the use of MaxCyte electroporation for the rapid generation of high-yield stable CHO cell lines to bridge the gap between early and late stage antibody development activities.
Has Your Current LIMS Implementation Been a Nightmare?
Because current traditional LIMS have not delivered on their promise, many organizations are still searching for solutions to optimize their laboratory operations. For those engaged in deploying traditional LIMS, frequent sleep-disturbing issues include poor flexibility and configurability, expensive and time-consuming customization, difficulties extending and upgrading systems, poor usability, lack of modular functionality, poor service/support, problems integrating with existing instrumentation/IT systems and extra time and resources required to meet critical qualification/compliance requirements. Learn how you can avoid the top 5 LIMS nightmares and rest easier with today’s next-generation process and execution-centric LIMS.
Polishing of Monoclonal Antibodies Using Capto™ adhere ImpRes in Bind and Elute Mode
MAbs and MAb conjugates are today in great demand for use as biopharamaceuticals. As a result, more cost-effective, efficient, and flexible process purification schemes are one of the highest priorities for MAb manufacturers. In this work, results from two case studies using Capto adhere ImpRes, a multimodal anion exchanger designed for polishing, are presented. Two different MAbs were purified in bind and elute mode. The results show high yields of MAb monomers, good clearance of aggregates, HCP, and leached protein…
Going Paperless from Lab to Plant
The drive to “go paperless” is a strategic initiative that offers demonstrable operational benefits in improving productivity, reducing cycle times and leveraging experimental and operational data along the entire research-development-manufacturing continuum. This technology brief reviews recent initiatives by science-based organizations to develop and deploy software that spans the lab-to-commercialization lifecycle using data standardization/harmonization technology and electronic lab notebooks (ELNs) to streamline technology transfer. Organizations adopting these proven informatics solutions can experience: Enhanced productivity Faster time to market Improved compliance More…
Supporting Advances in MAb Process Development and Manufacturing
Learn how GE Healthcare Life Sciences is supporting advances in MAb process development and manufacturing, and looking ahead, to Fabs and other antibody fragments that hold promise for new and more effective therapies.
Ten Tips for Single Use Pharmaceutical Tubing Selection
Tubing for pharmaceutical and bioprocess applications has requirements involving sterilization, extractables, ingredients, flexibility, and performance. This paper offers suggestions on how to narrow your selection.
The “early health” Vision
New approaches to vaccine production are needed. GE Healthcare Life Sciences contributes to the “early health” vision by supporting advances in vaccine development through innovative manufacturing solutions that help to shorten time-to-market, increase output, and assure quality.
Production of CGMP-Grade Lentiviral Vectors
Lentiviral vectors are important tools for gene transfer because of their ability to transduce a number of cell types without the need for host cells to be dividing. As a result, investigators are using them as gene delivery vehicles in clinical applications. Since lentiviral vectors play such a vital role in gene therapy, they need to be manufactured at large scale for clinical trials. But, large-scale production using CGMP methods can present a number of challenges.
To address these challenges, the authors of this case study developed a process that allows for extensive scale-up in a safe, sterile, and reproducible system to produce clinical-grade lentivirus. This manufacturing process is very efficient and can be carried out using minimal staff (two operators for production of each subbatch). It provides the extensive scale-up capacity necessary to produce CGMP-grade lentivirus, and it has been used successfully in several completed and on-going phase 1–2 ex-vivo gene therapy clinical trials.