When developing a biopharmaceutical manufacturing process, an R&D or process development team may fall into the trap of developing a process in the lab which is difficult to scale to work with larger volumes in production, leading to delays and costly additional development later on. It is therefore crucial that, where possible, technology is used at small-scale which can be scaled alongside the process in a consistent manner. Taking a thoughtful and strategic approach can help biopharmaceutical products get to…
BPI White Papers
A Smart Path for Novel Biologics
Roche Pharma Research and Early Development (pRED) was faced with a challenge: double the number of projects in process development without significantly increasing headcount. Pawel Linke, a pRED lab automation specialist, tells the story of how the group approached this challenge with a focus on obtaining high quality data through automation and by integrating independent lab systems and devices to streamline their workflow. Implementation of high-throughput process development systems requires an orchestrated approach of both hardware and software solutions, so…
Embedding Your Drug Strategy Within a Solid Foundation for Success
Demand for drugs and therapeutics is growing thanks to the globalization of pharmaceutical-based medicine. Manufacturing new drugs and getting them to market faster, more economically and safely demands development strategies and business models that support successful outcomes for both investors and patients. Outsourcing has become an increasingly attractive business model for pharma as companies seek partners who can deliver comprehensive end-to-end drug substance and drug product development. It has prompted contract manufacturing organizations (CMOs) to consolidate and expand to create…
Writing the Future of Biologics to Discover and Optimize Antibodies
With our unique DNA writing technology, Twist Biopharma is accelerating the way our partners discover and optimize antibody therapeutics. We have decades of synthetic antibody library design and selection expertise and have the ability to write any DNA sequence to enable faster, better discovery and development. Using our precise, rational, expertise in library fabrication, we can create libraries in a fundamentally different way that allow us to address tough targets, e.g. GPCRs. Since we are a DNA product company, we…
Confidence in Host Cell Protein (HCP) Coverage Assays with Differential Gel Approaches
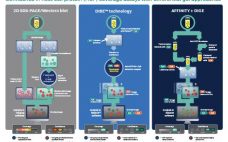
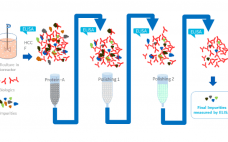
Enzyme-linked immunosorbent assays (ELISAs) are critical to detecting and removing host cell proteins (HCPs), a primary source of impurities in biologics development. Scientists must validate HCP ELISAs to ensure patient safety and meet regulatory guidelines – but different coverage assays come with different challenges, limitations, and benefits. The US Pharmacopeia recommends 2D differential gel electrophoresis (DIGE) combined with Western blot or immunoaffinity approaches, and labs might not be able to gain the full benefits of DIGE without significantly altering current…
Why, Why, Why… ELISA? A Look at the Benchmark HCP Assay
Host cell proteins (HCPs) are a primary source of impurity in biologics manufacturing. When present in drug formulations, HCPs can reduce efficacy, introduce toxicity, and increase risk of long-term immunogenicity. Understanding HCP profiles and integrating effective removal strategies are critical when developing a new biological drug, both for ensuring patient safety and fulfilling regulatory guidelines. HCP populations can be complex and structurally diverse, and most changes in upstream culture conditions affect HCP concentrations and control strategies. Accurate and reliable HCP…
Cyclic Cell Harvest with CONTIBAC® SU Filters
As the Biotech industry is moving towards single-use components, larger batch volumes and higher cell concentrations, conventional cell harvest technologies reach their limit. Depth filters, for instance, can only cope with the increasing demands by stacking more filter elements and therefore increasing the footprint and their economic burden. Hence, innovative solutions are needed to keep pushing the boundaries of the biologics production. The CONTIBAC® SU filter of DrM excels where existing technologies crumble. Unlike in any competing technology, the filtration…
Challenges Of Host Cell Protein Analysis With ELISA And How To Obtain Better Coverage Of Your ELISA Antibody Reagents
Why is host cell protein (HCP) detection and removal so critical when manufacturing biologics? Product purity and, most importantly, patient safety depends on it. Sandwich ELISA has been a favored method for HCP testing due to its high sensitivity and high throughput, but challenges and limitations exist. Coverage depends strongly on the use of antibody reagents that adequately detect and capture HCPs. In this webinar, we address effective techniques for both optimizing HCP ELISA coverage through the selection of reagents…
Effectively Securing Cell and Gene Therapies with Closed Systems
With their innovative new treatments, cell and gene therapies (CGT) are growing rapidly. But their traditional production processes don’t allow the supply of these therapies to keep up with demand. Historically, these therapies are produced for small patient populations in clinical trials using laboratory scale equipment and utilizing manual, open processes completed under laminar hoods. But manufacturers looking for more efficiency and flexibility are turning to new solutions. One key area of interest is the implementation of a closed system…
Understanding and Controlling Raw Materials Variation in Cell Culture Media
The FDA requires biopharmaceutical manufacturers to understand and control sources of variation according to the risks they present to process and product. Cell culture media and feeds have been shown to affect cellular performance, product quality, or both. The evolution of media formulas to those that are serum-free and chemically defined poses both a challenge and an opportunity. The challenge is identifying which of the many components have the greatest impact on cells and the molecules they produce. The opportunity…