The merging of Informa’s KNect365 division with pharmaceutical events operated by UBM brings another opportunity for BPI’s successful “BPI Theater” program: the “BioLIVE” theater taking place at CPhI. Our first foray into the theaters — our own speaker/roundtable sessions held in the exhibit halls of large events — was at a BIO International Convention some years ago. Through our relationship with the Biotechnology Innovation Organization, that came about to bring more technical content into the big event. As BIO grew…
March 2019
Frameworks and Strategies for Commercialization Success in the Biopharmaceutical Ecosystem
The “biopharmaceutical ecosystem” is a multibillion-dollar industry that encompasses large and small drug companies; ancillary providers of services, technologies, equipment, and infrastructure support; and tertiary groups that provide policy direction, regulatory standards, incubator space, and more. This ecosystem is vast and dynamic, evolving constantly as new ideas take hold to push the industry in new directions and present new opportunities for innovation and commercialization. However, many companies operating within this ecosystem struggle to understand their development and commercialization strategies, and…
China Can Be Ignored No Longer: Long-Term Biopharmaceutical Opportunities Based on Near-Term Demonstrated Growth
China has long served as “the world’s factory,” but many experts in the biopharmaceutical industry have assumed that making consumer electronics, clothing, and toys for a global consumer base does not translate well to making complex biologics on the global stage. However, according to a newly released report, the biopharmaceutical market in China reached a value over US$9 billion in 2018, with the domestic monoclonal antibody (MAb) market there making up a very large percentage of that (1). Much of…
Recommended Practices for Assuring Integrity of Single-Use Systems
The increasing uptake of single-use technologies (SUTs) in critical current good manufacturing practice (CGMP) processes and applications has made their integrity a critical quality attribute (CQA) for both suppliers and end users of such systems. Current regulations focus on final packaging, however, without taking into account the unique aspects of assemblies used in bioproduction. Ongoing initiatives include revision of PDA TR 27 (1) and creation of A STM workstreams (2, 3) to propose good practices for the integrity of single-use…
Points to Consider in Quality Control Method Validation and Transfer
The concept of an analytical lifecycle has been well received in the biopharmaceutical industry. In 2016, the US Pharmacopeia (USP) advocated for lifecycle management of analytical procedures (1) and defined its three stages: method design development and understanding, qualification of the method procedure, and procedure performance verification. The US Food and Drug Administration (FDA) has published guidance on process validation with a similar division into three stages: process design, process performance qualification, and process performance verification (2). For a manufacturing…
Apparent Matrix Effects in an Iduronate 2-Sulfatase Specific Activity Assay
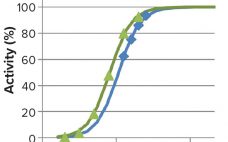
The recombinant fusion protein SHP631 consists of a chimeric monoclonal antibody binding to human insulin receptor and iduronate-2-sulfatase (I2S). This product is being developed as an enzyme replacement therapy to treat cognitive symptoms of Hunter’s syndrome. Because the current therapy (idursulfase, brand name Elaprase from Shire) cannot cross the blood–brain barrier (BBB), SHP631 is being developed to do so, enabling the presence of I2S in the brain. The enzymatic activity of this molecule is measured using the substrate 4-methyl umbelliferyl-α-L-idopyranosiduronic…
Developing an End-to-End Scale-Down Model for a Commercial-Scale Downstream Process: Enhancing Technology Transfer Efficiency
Large and complex protein molecules used as therapeutic agents are manufactured in a series of process steps that start with thawing of cell-bank vials and finish with filling and packaging (Figure 1). The cost and complexity of commercial-scale biomanufacturing processes make them prohibitive to troubleshoot or experiment at full commercial scale. Biopharmaceutical companies routinely use scale-down models (SDMs) of licensed commercial-scale processes to evaluate raw material changes, process improvements, and deviations (1) (Figure 2). Here, we outline some considerations in…
Smart Modular Package Units for Single-Use Processing: Addressing Cost, Speed, and Flexibility Challenges in Biologics Manufacturing
How to reduce costs, while still increasing speed, and flexibility in biologics manufacturing currently represent major issues for the biopharma industry. In this article, Burkhard Joksch, Product Manager Bioprocess Automation and Stuart Tindal, Product Manager FlexAct® Platform at Sartorius Stedim Biotech GmbH, detail the evolution of automation for single-use technology towards modular packaging units. They also including case studies of how these units can be used in real life cGMP processes, as well as explain the manufacturing and industry benefits…
Immunotherapy for Cancer: Why Fight Only Half the Battle?
Immunotherapy has made incredible strides over the past several years. Studies have shown it to be effective in treating many diseases that attack the immune system, such as multiple sclerosis, rheumatoid arthritis and psoriasis, and (most recently) cancer. With the latter in particular, although immunotherapy is proving to be effective for some patients, most do not respond to the treatment. It is time for the industry to reevaluate how it approaches immunotherapy especially for treatment-resistant patients. In the fight against…