The biopharmaceutical industry now incorporates single-use (SU) technology and systems in most production processes based on cell culture (1, 2). Implementation of such technologies has led to the availability of prepackaged and sterilized systems complete and ready for use with preinstalled mixers and monitoring probes. From upstream process- material preparation through final-product formulation, biopharmaceutical sponsors are increasingly presented with numerous SU solutions that support all major production platforms (3–5). The number of SU materials and suppliers in biopharmaceutical manufacturing has…
Supply Chain
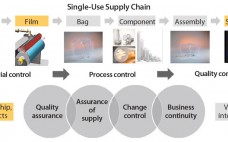
Enhanced Assurance of Supply for Single-Use Bags: Based on Material Science, Quality By Design, and Partnership with Suppliers
Growing adoption of single-use bags in commercial production of biopharmaceutical drugs raises new challenges for bag suppliers and drives the need for consistent product quality, improved assurance of supply, robust change management, and business continuity planning. In close collaboration with resin and film suppliers, polymer scientists and biologists at Sartorius Stedim Biotech have followed a stringent material science and quality by design (QbD) program to develop a completely new polyethylene film and to achieve consistent performance of new Flexsafe bags…
A Critical Mission: Clinical Trial Material Storage and Distribution
As if manufacturing of investigational medicinal products (IMPs) weren’t challenging enough already, the appropriate storage and distribution of sensitive biological products can be an adventurous journey itself — especially if not carefully planned and managed. No one solution fits all situations. Many things must be evaluated during planning stages. For example, what is more important: time to delivery or quality of transport and product integrity until drug can be administered to a patient? It is important to understand key storage…
Supply Chain Challenges in the Biopharmaceutical Industry: A Case Study Following the 2011 Tsunami in Japan
Global manufacturing of biopharmaceuticals for human use helps save the lives of millions of people and is a large commitment to public health. The industry operates in an environment with financial uncertainties and complex international supply chains, so the question of risk mitigation is paramount. There is an expectation that comprehensive risk mitigation programs should be in place to minimize the risk of supply chain interruptions that would negatively affect the manufacture of these vital therapeutics. Here we share how…
Managing Contract Relationships with Quality Agreements: Keeping in Mind the New FDA Guidance
Using contract manufacturing organizations (CMOs) to augment your supply chain is not a new phenomenon in the pharmaceutical industry. One of my first projects in industry involved developing a process for a recombinant protein while manufacturing materials for clinical trials. My team recognized that the company did not have the money to build a plant for manufacturing an unproven product, and it was not bullish to the risk of investing, so we turned to a contract manufacturer in Austria. That…
Cell Therapy Bioprocessing Technologies and Indicators of Technological Convergence
The cell therapy industry is undergoing a natural evolution from scientific curiosity into a commercially and clinically attractive opportunity (1). This evolution is by no means complete, and growing evidence suggests that its progression is driving significant developments in cell therapy bioprocessing — notably, convergence. Table 1: 194; () Progressively, bioprocessing technologies primarily used in production of noncell-based products are being evaluated for cell therapy bioprocessing applications (2). Consequently, this process of convergence is leading to an increasing proportion of…
Powders and Bulk Liquids
The two major bioprocess fluids — culture media for upstream production and buffers for downstream processing — are classic single-use products. They are used once and then disposed of. The two basic options for both differ by physical state: powdered media and buffers (“powders” for in-house preparation of liquids by end users) and bulk liquid culture media and buffers, which are fully prepared by their suppliers (“liquids”). We conducted market research studies comparing the benefits and risks (value…
Innovation in Biopharmaceutical Manufacture
The following is a report from a workshop on innovation in biopharmaceutical manufacturing held at the Annual bioProcessUK Conference in Bristol on 29 November 2012. The aim of the workshop was to access the experience of practitioners in the United Kingdom so as to understand better the challenges and opportunities for innovation in this sector. The workshop addressed the drivers that influence the implementation of process improvements and novel technologies in biopharmaceutical manufacture from the perspective of both manufacturers and…
Container–Closure Integrity
An increasing number of biopharmaceuticals — including vaccines, stem cells, and proteins — require cold storage to maintain efficacy before use. However, the ability to maintain container–closure integrity (CCI) during cold storage is not completely understood. Concerns about CCI failure have been raised for storage and shipment of such products in rubber-stoppered vials under cold conditions (e.g., −80 °C or on dry ice). Commonly used butyl stoppers are believed to lose their elastic properties below their glass transition temperature (Tg),…
Preparedness Ahead of Pandemic Outbreaks
Lively debate in 2012 concerned the risks and benefits of laboratory studies that created a contagious H5N1 avian pandemic influenza (flu) laboratory-strain virus. One benefit of the public debate is that it reminded governments of the increasingly likely and disastrous possibility of a devastating flu pandemic on the scale of the Spanish influenza outbreak of 1918. Natural evolution of circulating H5N1 viruses could lead to emergence of a deadly and contagious strain (1). Here we outline conventional flu vaccine options…