Single-use technologies have transformed biopharmaceutical manufacturing by providing tremendous and proven opportunities to reduce costs, improve flexibility, and shorten cycle times. The expansion of such technologies into commercial production has naturally raised new challenges for both end users and suppliers, thus driving the need for a critical look at risks associated with their use. End users now face a new challenge: how to assess their own supply chains for robust assurance of supply. What is the suppliers’ responsibility in addressing…
Supply Chain
Addressing Variability in Product Labeling: Explore Dynamic Labeling for Your Global Enterprise
Adding to the complexity of drug-product labeling, companies face a broad range of evolving requirements. Those include regional, language, customer, and regulatory requirements that must be met quickly and efficiently to prevent supply-chain disruption. Companies that cannot meet those requirements can end up with fines, dissatisfied customers, and loss of business. Enterprise labeling solutions allow drug makers to deal with variability in labeling by providing label formatting that supports myriad different label combinations with a minimum number of label designs.…
Clinical Supply Chain: A Four-Dimensional Mission
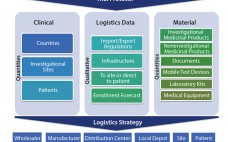
A clinical supply chain fulfills perfectly all four characteristics of what Packowski describes as a “VUCA” (volatility, uncertainty, complexity, and ambiguity) world (1). In commercial markets, supply chains depend predominantly on consumer orders. For global drug development programs, both investigators and patients can be considered end consumers. The international journey of a specific investigational medicinal product (IMP) includes all of the following: global sourcing of comparators, manufacturing, storage, distribution, site/patient (consumer) management, and return and destruction of the IMP. Application…
Standardization of Disposables Design: The Path Forward for a Potential Game Changer
Recent articles have described how the debate on standardization is slowing down adoption of single-use technology (1). The Standardized Disposables Design (SDD) initiative is working to design simple standard single-use solutions for real-life examples (e.g., buffer bags). In reality, a buffer is a buffer whether it is made in Europe, Asia, or America, so in essence different solutions are not necessary for different end users. A buffer bag is not difficult to design, and it does not vary greatly in…
A Risk-Based Approach to Supplier and Raw Materials Management
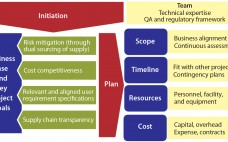
Ensuring a continuous supply of safe medicines is a key objective for the pharmaceutical industry and health authorities alike. A critical component to that end is maintaining a reliable supply of qualified raw materials (RMs) used in drug production. However, changes in suppliers, their processes, their providers, and consequently the materials they supply can occur (for a number of reasons) at any time during the life cycle of drug production. A product-supply organization therefore must be prepared to address such…
The Cell Therapy Supply Chain: Logistical Considerations for Autologous Immunotherapies
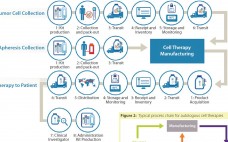
Among the basics of building a successful logistics strategy for the management of cell-based material, some better-known and important factors to consider include selecting the right dry-shipping unit, qualifying that container for a particular payload and shipping configuration, choosing an appropriate data logger, creating a chain of custody, evaluating a transit carrier, and anticipating potential problems inherent in shipping at cryogenic temperatures (1). Here, I’d like to go beyond those basics to address some lesser-known considerations. These factors may be…
Bioreactor Design for Adherent Cell Culture: The Bolt-On Bioreactor Project, Part 4 — Process Economics
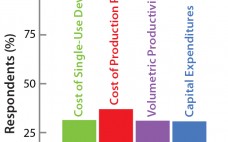
The Bolt-on Bioreactor (BoB) project is an independent initiative developing and commercializing a bioreactor for efficient, automated culture of adherent cells for biopharmaceutical applications (1). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges: volumetric productivity (2), process automation (3), containment and sterility (4), and process economics. This month concludes a four-part series addressing each of those challenges while describing design features…
Exploring Options for Dual Sourcing of Single-Use Components
As the bioprocess industry progressively adopts single-use technologies for large-scale manufacturing (1, 2), biomanufacturers’ increased reliance on integrators for critical production equipment continues to raise concerns about supply chain security. The need to mitigate risks associated with the supply of single-use components (e.g., bioreactors, aseptic connectors, tubing, filters) has led to growing interest in the dual sourcing of those materials. To that end, integrators and end users alike are exploring the definition of functionally equivalent products, how functional equivalency can…
Shrink Your Inventory Costs And Make Your Staff Happier
Shire’s process development department recently overhauled its inventory control system. The result was a projected five-year net benefit of over US$1.5 million and an immediate increase in its scientists’ productivity and satisfaction. Hiding in Plain Sight We asked one of our scientists why he kept 12 cases of gloves on his laboratory bench. “I use a lot of them,” he told us. “I don’t want to run out.” When asked how long his supply would last, he replied, “I don’t know.…
Creating Value Through Investment
During my MBA course, Professor Pierre Casse — then at the International Institute for Management Development (IMD) in Lausanne, Switzerland — regularly reminded us that one key to success was constantly finding new ways to “delight and inspire your clients” by creating value. SAFC achieved that objective in its “Overcoming Supply Chain Vulnerability and Lowering Risk in Biopharmaceutical Manufacturing” symposium 17–18th June 2014 in Turnberry, Scotland. Along with a day of industry insight, the event included a visit and tour…