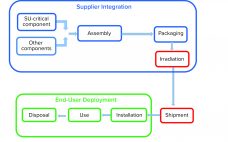
Advanced therapy medicinal products (ATMPs) hold much potential for improving healthcare. They offer hope for treating or even curing patients. The biopharmaceutical industry has recognized the importance of making such therapies accessible to as many people as possible. To provide personalized ATMPs, biomanufacturers are shifting toward flexible, patient-centered production processes. A Paradigm Shift in ATMP Manufacturing Ex vivo cell and gene therapies are particularly promising approaches to personalized regenerative medicine. Thus, it is no surprise that the numbers of US…
Single Use
Increasing the Integrity of Closed Systems: Advantages Offered by Single-Use Connectors
Due to the complexity of biologic development and manufacturing and the business pressures of the biopharmaceutical industry’s landscape, the margin for error in today’s industry is small. That is why minimizing the threat of contamination is critical when using a closed system for drug development and manufacturing. Yet the traditional method of connecting each step in a closed process can present other risks to the integrity of your product. Therefore, it is important for you to be confident in selecting…
Analyzing Single-Use Polymers for Cell Culture Processes: Comparison of Cell Growth and Viability Test Procedures
The acceptance and implementation of single-use systems (SUS) or “disposables” has increased strongly in bioprocess development and biopharmaceutical manufacturing over the past two decades. Typically, suppliers provide SUS presterilized and ready to use. Using SUS eliminates time-consuming and expensive cleaning procedures (which often require corrosive chemicals and a large amounts of water) and removes the need to perform cleaning validation between batches. The application of SUS reduces the risk of product cross-contamination and increases product and patient safety (1–5). Polypropylene…
Facilitating Workforce Development: A case study on improving single-use training through vendor and end-user collaboration
Discover how Pall Corporation and Lonza collaborated to improve single-use technology training for operators using a blended approach to learning. This article presents: The importance of SUT training for operators. Why a blended approach ensures that operators get the training they need in the format that best suits their learning style. How collaboration between suppliers and biomanufacturers can shorten training program development timelines and increase the quality of training tools. How Pall and Lonza developed a digital training approach together.…
The Green Imperative: Part 3 — Postuse Management of Single-Use Bioprocessing Materials, Today and Tomorrow
The world desires a more sustainable economy in which resources can be saved, products can be profitably used, and at the end of their useful life, component materials can be recycled into other useful products. The bioprocessing industry has made efforts to meet those goals and has learned a great deal about the role of plastic components in sustainable manufacturing. The most important lesson might be that a science-based approach is required to provide an accurate benchmark of manufacturingĘĽs environmental…
Integrity of Single-Use Systems: Practical Applications and Deployment
Single-use (SU) technology plays an important role in modern vaccine and biologics manufacturing. System integrity, managed by critical process controls, ensures sterility and is a prerequisite for successful leak-free processing. Nonintegral systems cause loss of product, quality, and time; increase costs through investigations; and lead to potential safety problems. The BioProcess Systems Alliance (BPSA) issued a white paper in 2017, Design, Control, and Monitoring of Single-Use Systems for Integrity Assurance (1), that describes in detail the strategies for design and…
Single-Use Technology for Formulation and Filling: A Case Study from Swissfillon AG and Pall Corporation
Swissfillon AG is a contract manufacturing organization (CMO) based in Switzerland. Fully compliant with current good manufacturing practice (CGMP) regulations, it provides state-of-the-art aseptic filling for pharmaceutical and biotechnology companies, from clinical-phase materials to commercial quantities. This CMO specializes in high-value, difficult-to-fill products. Swissfillon recognized that adoption of single-use systems (SUS) on a commercial scale required major improvements in consistency and reliability compared to manual operations at pilot and clinical-trial scale. The single-use formulation and filling process, which includes an…
Design and Performance of a New Single-Use pH Sensor with Long Shelf Life and High Stability
Single-use biopharmaceutical manufacturing systems require gamma-sterilizable, highly stable, accurate, and simple-to-use single-use pH sensors with a long shelf life. Herein we report the design and performance of a single-use pH sensor technology optimized for single-use bag applications such as those found in bioreactor and mixing applications. This technology is the basis of Emerson’s Rosemount 550pH Single-Use Sensor. The sensor is compatible with gamma irradiation and can be attached to a single-use bioreactor bag via industry accepted ports. With the incorporation…
Single-Use Systems for Storing and Shipping Frozen Drug Materials
Using presterilized, single-use freeze–thaw systems instead of traditional freeze–thaw platforms that include stainless-steel tanks and bottles can help biomanufacturers manage the quality of their drug substances. Single-use assemblies reduce the risk of cross-contamination, simplify dispensing, and decrease the number of manual interventions during freezing, thawing, handling, and shipping. However, implementing a freeze–thaw process requires careful testing of the physical and thermal properties of single-use systems and related aseptic connectors as well as assessment of drug-substance quality and stability. Such evaluation…
Benefits of Single-Use Standardization: Adopting a Standard Design Approach
It is widely accepted that standardization of single-use designs and assemblies would be beneficial to the biopharmaceutical industry, providing it quickly with simple and economical solutions. Meanwhile, as implementation of single-use technology increases across the biopharmaceutical industry, suppliers are struggling to keep up with demand. That has been evident particularly in current supply issues caused by the COVID-19 pandemic. A widely adopted single-use standardization approach could help alleviate such supply issues. That would not only benefit the industry by helping…