Monitoring critical process parameters (CPPs) and key performance indicators in bioreactor control systems is crucial to ensure proper cell growth and protein production. Today, most of the major biopharmaceutical companies employ capacitance measurement, in R&D and through process development to manufacturing. Owing to the increased use of single-use bioreactors and building on Aber’s experience with single-use capacitance sensors, the latest Futura neotf single-use capacitance sensors have been specifically developed for integration into Thermo Fisher Scientific bioprocess containers (BPCs) for use…
Process Monitoring and Controls
Measuring Cell Density in HyPerforma S.U.B.s with ABER Futura neotf
Emerging Strategies for Drug-Product Comparability and Process Validation: Part 2 — Validation, Legacy Products, and Lifecycle Management
This two-day CASSS CMC Strategy Forum explored many technical, practical, and regulatory facets of biological drug-product (DP) analytics, process validation, and comparability. Part 1 of this report summarized the discussions on drug-product analytics and comparability in BPI’s March 2021 issue (1). Here we report on day two presentations and discussions on validation, legacy products, and lifecycle management. Session Three: Drug-Product Validation The morning session focused on principles of process validation with examples of challenges specific to drug products. New Risk-Based…
Using Prior Knowledge to Estimate Long-Term Variation
A reasonable estimate of long-term variation for a biopharmaceutical product critical quality attribute (CQA) can be challenging to justify, especially in the early stages of a product’s lifecycle when only limited data are available. However, if the combination of product and analytical method reasonably can be matched with historical data, prior knowledge can provide an estimate of a value. This variation estimate could be used to assist in risk assessments related to continued process verification (CPV) activities, including control charting…
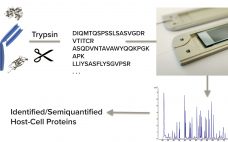
Nontargeted HCP Monitoring in Downstream Process Samples: Combining Micro Pillar Array Columns with Mass Spectrometry
Protein biopharmaceuticals have emerged as important treatments for diseases with otherwise unmet medical needs. These biologics are produced by recombinant mammalian, yeast, or bacterial expression systems. Along with therapeutic proteins, those cells produce endogenous host-cell proteins (HCPs) that can contaminate biopharmaceutical products despite multiple purification steps in downstream processing. Because such process-related impurities can affect product safety and efficacy, they need to be monitored closely. Multicomponent enzyme-lined immunosorbent assays (ELISAs) presently are the workhorse method for HCP testing, with high…
Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies, Part 2: Process Modeling and Analytics
Part 1 of this article focused on the first two sessions of a CASSS chemistry, manufacturing, and controls (CMC) forum entitled “Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies,” which took place on 16–17 July 2018 in Gaithersburg, MD. Those sessions focused on upstream and downstream process technologies and strategies (1). Part 2 highlights the final two conference sessions on process modeling, control, and analytics. Process Modeling and Control The third conference session was “Modeling and Control Strategies.” Advancements in…
Product Quality Attribute Shifts in Perfusion Systems, Part 2: Elucidating Cellular Mechanisms
Part 1 of this two-part report describes an investigation into the potential cause(s) and ways to control a product quality attribute (PQA) of a protein expressed in perfusion cell culture (1). The presence of low–molecular-weight (LMW) species following size-exclusion high-performance liquid chromatography (SEC-HPLC) is a protein quality attribute that can indicate an increase in truncated forms of the expressed protein and/or other LMW moieties. The expressed protein in this study is a heavily glycosylated recombinant glycoprotein (rGP) comprising two subunits:…
Practical Considerations for Statistical Analyses in Continued Process Verification
Several statistical techniques can be used to assist in monitoring biopharmaceutical product quality attributes as part of continued process verification (CPV) activities. These include run charts, control charts, and capability analyses. Below, I provide an overview and recommendations on statistical strategies when developing a CPV program, considering the expected behavior of manufacturing results in the biopharmaceutical industry. Presence of Autocorrelated Data In a previous study, I highlighted the tendency for data to be positively autocorrelated (values are closely related to…
Ultrasonic Flow and Bubble Sensors: Optimize Process Quality in Single-Use Bioprocessing Applications
Process monitoring in laboratory and production environments enables continuous control and optimization of critical process parameters. Hence, the early detection of errors is an effective means of increasing process efficiency and reproducibility, improving quality and safety parameters, and reducing long-term costs. Highly precise and flexible noncontact clamp-on flow and bubble sensors are useful instruments to fulfill these goals. They can be applied effectively to buffer and media preparation, chromatography and filtration systems, bioreactors and fermentors, feed and transfer lines, and…
Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies, Part 1: Upstream and Downstream Strategies
Future biomanufacturing must address industry drivers, including the need for decreasing cost of goods (CoG), increasing market globalization, shortening development time for pipeline products, reducing risk to patient supply, and improving product quality. A CASSS chemistry, manufacturing, and controls (CMC) forum entitled “Next-Generation Biotechnology Product Development, Manufacturing, and Control Strategies” took place on 16–17 July 2018 in Gaithersburg, MD, to address those opportunities. Advanced technologies include single-use bioreactors, alternating tangential-flow (ATF) systems used during fermentation, modular and closed process equipment,…
Run Rules with Autocorrelated Data for Continued Process Verification
Control charts can be used to assist in monitoring of biopharmaceutical product quality attributes as part of continued process verification activities. A number of tests known as run rules have been developed to assess whether biomanufacturing processes remain in statistical control. In practice, results for such attributes can be positively autocorrelated. Simulated data are used to assess the performance of run rules with autocorrelated data to assist in determining risk–reward profiles for process monitoring. Autocorrelated Data The tendency for data…