The product development team at a gene therapy contract development and manufacturing organization (CDMO) was working on a high-priority drug-substance project for a key client. The material was crucial to that client’s early stage clinical trial, with an immediate value over US$500,000 to both the client and the CDMO. Unfortunately, the bioreactor used in the upstream process — a transfection unit operation for an adenoassociated virus (AAV) vector — had developed an intermittent problem that could force it to shut…
Process Monitoring and Controls
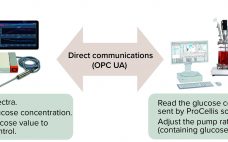
Automated Process Control Based on In Situ Measured Glucose Concentration
A process analytical technology (PAT) strategy involves defining critical process parameters (CPPs) of a biomanufacturing process that influence critical quality attributes (CQAs) and controlling those CPPs within defined limits. Doing that enables consistent product quality and helps companies reduce waste and costs. Glucose is an important CPP in bioprocessing and cell therapy. Glucose often is fed as a bolus addition based on daily off-line measurements, but that can lead to high glucose fluctuations and to excessive glucose feeding, which can…
Seamless Integration of Glucose Control: Using Raman Spectroscopy in CHO Cell Culture
The process analytical technology (PAT) and quality by design (QbD) guidelines promoted by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) support the idea that quality cannot be “tested into” a biologic product but must instead be part of its process design. Seamless integration of analytical data with bioprocess monitoring and control is crucial to understanding a process and overcoming manufacturing challenges that arise in the course of development. Monitoring of product quality attributes (PQAs)…
Implementation of Established Conditions: Learnings from a “Sharing Science Solutions” Workshop
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline Q12 (1) (step 4 sign-off in November 2019) is in the process of being implemented in a number of regulatory regions. The document provides additional frameworks for pharmaceutical life-cycle management. It is intended to support globally harmonized regulatory tools such as established conditions (ECs) and product life cycle management (PLCM) documents to facilitate postapproval changes to chemistry, manufacturing, and controls (CMC). Although a harmonized framework…
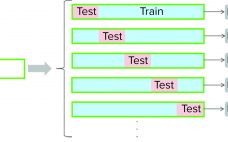
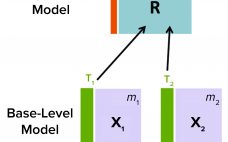
Multivariate Data-Driven Modeling for Continued Process Verification
Continued process verification (CPV) is an integral part of process validation for the manufacture of human and animal drugs and biological products (1). It is designed to meet three primary goals: maintain a validated state of products, their processes, and related systems; enable continuous process improvements; and meet regulatory requirements for life-cycle validation. A CPV program for a biologic product entails regular collection of data related to critical process parameters (CPPs) and critical quality attributes (CQAs) and the preprocessing, analysis,…
Advanced Data-Driven Modeling for Biopharmaceutical Purification Processes
Purification is an essential process in biopharmaceutical manufacturing that separates a therapeutic protein in its active form from impurities. A typical purification process consists of several chromatography unit operations, and each unit operation comprises multiple phases. During the operation of each step, continuous (time-series data per parameter for each batch) and batch data (one data point per parameter for each batch) are generated by in-line sensors installed in chromatography skids on the production floor and with at-line/off-line in-process samples, respectively.…
Design and Performance of a New Single-Use pH Sensor with Long Shelf Life and High Stability
Single-use biopharmaceutical manufacturing systems require gamma-sterilizable, highly stable, accurate, and simple-to-use single-use pH sensors with a long shelf life. Herein we report the design and performance of a single-use pH sensor technology optimized for single-use bag applications such as those found in bioreactor and mixing applications. This technology is the basis of Emerson’s Rosemount 550pH Single-Use Sensor. The sensor is compatible with gamma irradiation and can be attached to a single-use bioreactor bag via industry accepted ports. With the incorporation…
Clamp-On Flow Meters for Process Monitoring
Process monitoring entails systematic recording or measurement of an operation or process by means of technical aids. Repeated, regular execution is a central element of that activity. Statistical process control and management help to optimize and stabilize processes. They also ensure appropriate monitoring of threshold values. Ultrasound-based clamp-on flow meters are ideal measuring methods for that purpose because they enable data collection without requiring operators or equipment to intervene in a process. Noncontact flow sensors also combine high precision with…
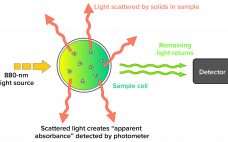
Validation of a Next-Generation Single-Use Turbidity System
Turbidity describes the relative clarity of a liquid as the result of suspended solids. Instruments that measure turbidity typically use a beam of light to detect particles by measuring the difference between the amount of light emitted from the light source and the amount that is received by a detector. Such measurements are affected by the size, shape, and number of particles in a sample of liquid because those solids scatter the incoming light, which provides an apparent absorbance that…
The Five Heresies of Cell Culture: Debunking Conventional Wisdom
Cell culture and bioprocessing conventional wisdom remains a hurdle for the wider adoption of more precise tools. It has been more than 60 years since any real progress has been made towards creating a more accurate and reliable way of performing cell culture monitoring to better understand the effects of things like pH and oxygen at the pericellular level. At SBI, we’re developing optical sensing technologies that unlock the “black box” of cell culture to bring actionable insights to scientists…