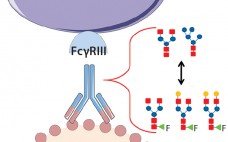
Product quality attributes are critical for the functionality and manufacturability of therapeutic antibodies. They can be significantly influenced by a number of production process parameters, such as cell culture media. The composition of growth and feed media can influence antibody glycosylation, including the concentration of ammonia, glutamine, glucose, and metal ions (1, 2). Thus, it is critical during media development and optimization to monitor and consider a culture medium’s impact on glycosylation. For therapeutic antibodies whose mechanism of action includes…
Manufacturing
Rapid Formulation Development for Monoclonal Antibodies
Monoclonal antibodies (MAbs) are at the focal point of biologics development. Many of the best-selling drugs are therapeutic MAbs or related proteins (1–2). The combined world-wide sales from MAbs will be nearly US$125 billion by 2020 (3). About 50 MAb products treating a range of diseases have been approved in the United States or Europe. With the large number of MAbs progressing through discovery, biomanufacturers need to accelerate process development and move projects rapidly into clinical manufacturing (4–5). Formulation development,…
Addressing Variability in Product Labeling: Explore Dynamic Labeling for Your Global Enterprise
Adding to the complexity of drug-product labeling, companies face a broad range of evolving requirements. Those include regional, language, customer, and regulatory requirements that must be met quickly and efficiently to prevent supply-chain disruption. Companies that cannot meet those requirements can end up with fines, dissatisfied customers, and loss of business. Enterprise labeling solutions allow drug makers to deal with variability in labeling by providing label formatting that supports myriad different label combinations with a minimum number of label designs.…
The Global Emergence of Single Use
Adoption of single-use manufacturing continues to expand globally and is showing no signs of slowing down. And why should it? The biopharmaceutical industry is challenged to produce safe, effective therapies and vaccines amid the constant pressure to lower cost per dose in addressing healthcare needs of not only the western hemisphere, but emerging markets as well. To meet these challenges, manufacturers are turning to single-use and hybrid systems that incorporate a balance of stainless and single-use equipment. Over the past…
A Single-Use Process for Production of Recombinant Human Follicle-Stimulating Hormone
Follicle-stimulating hormone (FSH) is a heterodimeric glycoprotein consisting of noncovalently linked α and β subunits. It stimulates the growth of immature follicles in ovaries and primary spermatocytes in testes and thus plays an important role in human reproduction (1). Human menopausal gonadotropin for infertility treatment was first introduced into clinical practice in 1950 (2, 3). Subsequently, treatments with urinary FSH have been replaced by recombinant human FSH (rh-FSH), which has been shown to provide several advantages such as absence of…
Pressure Rating for Bioprocess Single-Use Assemblies
Single-use systems (SUSs) are engineered process equipment solutions for pharmaceutical and biologics production. They offer several key advantages, such as lowering energy costs to reduce utility requirements, minimizing cleaning validation efforts, reducing water and chemical use, and enabling flexibility in manufacturing. Use of SUSs is increasingly popular in almost all fields of bioprocess applications (1, 2). SUSs most commonly comprise components made of polymeric materials, which together create a system or unit operation designed for one-time or campaign use. Single-use…
Monitoring Live Biomass in Disposable Bioreactors
Often simply referred to as capacitance, radio-frequency (RF) impedance has been used for over two decades to measure online biomass. It is generally regarded as the most robust and reliable method to monitor live-cell concentrations in mammalian cell culture (1). Many biopharmaceutical companies have now made the transition from conventional glass or stainless steel multiuse (MU) vessels to single-use (SU) bioreactors. Disposables are rapidly becoming the preferred platform for new processes requiring current good manufacturing practice (CGMP) compliance. At the…
Evaluating New Film for Single-Use Bags: Growth Performance Studies with Animal and Human Cells
In biopharmaceutical development and manufacturing processes, single-use technology has become widely accepted (1). Storage and cultivation bags are particularly common. They are fabricated from plastics consisting of multilayer films and are typically provided gamma-sterilized by suppliers (2). The bags offer several advantages such as savings in time and cost. Lowered contamination risk results from reduced cleaning and sterilization demands. However, some adverse effects of polymer films on cell growth and metabolism have been reported, both for storage and cultivation bags…
The Year of Data Integrity: 2015 Brought a Worldwide Focus on Training, System Design and Control, and Data Management
Each year, regulatory agencies from around the world focus on critical aspects of the pharmaceutical quality management system, bringing awareness to the industry and continuing to effect positive change. In the past five years, risk assessments, electronic records, and outsourced activities have been in the spotlight. As 2015 closed out, it was clearly the year of data integrity. In March 2015, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) published its GMP Data Integrity Definitions and Guidance for Industry,…
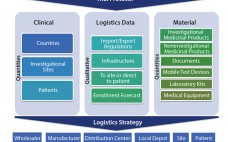
Clinical Supply Chain: A Four-Dimensional Mission
A clinical supply chain fulfills perfectly all four characteristics of what Packowski describes as a “VUCA” (volatility, uncertainty, complexity, and ambiguity) world (1). In commercial markets, supply chains depend predominantly on consumer orders. For global drug development programs, both investigators and patients can be considered end consumers. The international journey of a specific investigational medicinal product (IMP) includes all of the following: global sourcing of comparators, manufacturing, storage, distribution, site/patient (consumer) management, and return and destruction of the IMP. Application…