Monoclonal antibodies (MAbs) are the most prominent and successful therapeutic proteins in the pharmaceutical industry. More than 35 MAbs have been approved to treat a range of conditions, and hundreds more are in development (1, 2). Once, the upstream cell culture process was considered the bottleneck to producing high antibody doses required for treatment, but recent advances in cell culture technology have boosted antibody titers to the range of 5–10 g/L (3). That increase in productivity has shifted focus onto…
MAb
An Industrial Platform Solution for Antibody Fragment Purification
Compared with traditional approaches such as chemotherapy and radiotherapy, monoclonal antibodies (MAbs) have become the most successful cancer treatments in the past 20 years (1). With great clinical success in many therapeutic areas, MAbs now account for >40% of the entire biotechnology drug market, and sales are projected to be >US$160 billion over the next few years in the United States alone (2). More than 35 MAbs have been approved for clinical use, and hundreds more are filling industry development…
Improved Fluorescent Labeling Efficiency of N-Linked, High-Mannose Oligosaccharides: Using 8-Aminopyrene-1,3,6-Trisulfonic Acid (APTS) for Analysis of Glycoproteins
Glycosylation of proteins, including monoclonal antibodies (MAbs), is recognized as important for the efficacy, immunogenicity, antibody-dependent cell-mediated cytotoxicity (ADCC), and complement-dependent cytotoxicity (CDC) of biotherapeutics (1–6). So research and development of protein candidates is increasingly focused on the effects of glycosylation and how its pathway is affected in the Golgi system of cells involved in biosynthetic processes (7). Such attention on glycosylation has helped advance analytical technologies such as high-pH anion-exchange chromatography (HPAEC) (8); normal-phase chromatography (NP- HPLC), hydrophilic-interaction chromatography…
The Importance of the Concentration-Temperature-Viscosity Relationship for the Development of Biologics
JIM DELILLO (WWW.FREEIMAGES.COM) Patient preference and a competitive landscape in the parenteral market have fueled the need for convenient delivery systems and a desire for less‑frequent dosing injections. Monoclonal antibodies (MAbs) often have high dose requirements, so they must be formulated at very high concentrations (1). At low concentrations, an antibody solution’s viscosity increases moderately as a function of protein concentration. But at high concentrations (>100 mg/ mL, depending on the molecule), viscosity increases exponentially (2, 3). Thus, a specification…
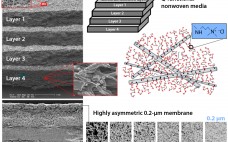
Characterization of Postcapture Impurity Removal Across an Adsorptive Depth Filter
In the manufacture of monoclonal antibodies (MAbs), the first purification step following harvest clarification is normally protein A affinity chromatography because of its high selectivity for IgG and high process yield (1, 2). At this stage, a MAb is eluted from a protein A ligand at low pH and then held or adjusted to a low pH (pH ≤ 3.8) for a given amount of time before pH adjustment, usually ≥30 minutes, in a virus inactivation (VI) step targeted at…
A Multidisciplinary Approach to Manufacturing Biotherapeutics
Optimizing antibody manufacturing processes has gone beyond the first-order goal of achieving elevated protein titers and now also focuses on understanding biologic and manufacturing process variables that define cellular machinery and protein quality. A holistic approach to biotherapeutic manufacturing incorporates several applied disciplines such as biology, engineering, process control, signal processing, and modeling to reduce the “black-box” model of cell- based protein production into an operational design space. This is in line with the US Food and Drug Administration’s quality…
Affinity Capture of F(ab’)2 Fragments: Using Twin-Column Countercurrent Chromatography
Antibody fragments are potent active drug substances (1–4). Because they lack glycosylation, they can be produced using different biological expression systems, including yeast and microbial systems as well as mammalian cells. These molecules are interesting as biopharmaceuticals because they are smaller than full-size antibodies and therefore may penetrate better into different tissues. Antibody fragments are cleared faster in biological systems because they lack the Fc antibody structural region (4). However, fragments may be conjugated to increase their size for improved…
Protein A Intermediate Wash Strategies
Protein A affinity chromatography offers efficient monoclonal antibody (MAb) purification and is used extensively in large-scale MAb production. As is the case with most chromatography media, protein A resins often have some degree of nonspecific binding, which causes host-cell proteins (HCPs) to coelute with a MAb. To reduce nonspecific binding interactions, an intermediate wash step can be performed before product elution. Doing so can improve product purity, extend column lifetime, and potentially eliminate a subsequent polishing step. For large- scale…
Immunoglobulin Fc-Fusion Proteins Part 2: Therapeutic Uses and Clinical Development
The potential therapeutic value of many proteins — including enzymes, receptors, cytokines, blood factors and peptides — can be realized by fusing them to the Fc region of human immunoglobulin G. Of the 46 monoclonal antibody (MAb) and MAb-derivative products approved by the FDA to date as human therapeutics, 10 are Fc-fusion proteins (Table 2). Among approved products, several structural variations are represented (Figure 4). In BPI’s October 2014 issue, Part 1 of this review examined the structure and manufacturing…
Immunoglobulin Fc-Fusion Proteins Part 1: Their Design and Manufacture
Over the past three decades, 45 monoclonal antibody (MAbs) and MAb-derivative products have been approved for therapeutic use in the United States (Table 1). One class of antibody derivatives is growing in importance: Fc-fusion proteins. Many biologically active proteins, including receptor ECDs (see “Abbreviations” box), cytokines, enzymes, and bioactive peptides have very short serum half lives because rapid renal clearance limits their exposure in target tissue (and, consequently, their pharmacological effect). The primary reason for fusing a biologically active protein…