As with many other aspects of biopharmaceutical development, evolving technologies are transforming drug-product development and manufacturing. In a November 2020 report on the Informa Connect âDrug Delivery Partnershipsâ meeting, BPI senior technical editor Cheryl Scott reviewed how new delivery-device technologies are bringing together companies with disparate expertise to serve patients with chronic conditions better than ever before. The ever-increasing capabilities of electronics and information technology (IT) not only are enabling development of âsmartâ delivery devices, but also improving the potential…
Formulation
Ask the Expert: Translating Inhaled and Nasal Technologies for Biologics Delivery
Gastric delivery is unachievable for most biopharmaceuticals, so drug developers formulate biologics primarily for intravenous infusion or injection. However, inhaled and nasal delivery options are attracting considerable attention because they enable targeted delivery of a wide range of therapeutic proteins. On 23 June 2020, Mark Parry (technical director at Intertek) presented an âAsk the Expertâ webinar that described critical considerations for inhaled and nasal delivery for biologics. Parryâs Presentation Because biopharmaceuticals are complex products, developers need compelling reasons to choose…
Ask the Expert: Ultrapure Gelatin Can Optimize Your Excipient Screening for Vaccine Formulation
In his 28 May 2020 âAsk the Expertâ presentation, Jeroen Geeraerts (business development manager at Rousselot) highlighted that, as of May 2020, 118 candidate vaccines for the novel coronavirus (SARS-CoV-2) had reached clinical or preclinical evaluation. Of those, 11 use inactivated and liveâattenuated approaches, which require strong stabilizing agents to maintain vaccine potency. Geeraerts explained why inactivated and liveâattenuated vaccines require stabilizing agents and why vaccine companies prefer pharmaceutical-grade gelatin for such applications. Next, Geeraerts described how his companyâs X-Pure…
eBook: Formulation, Fill, Finish â â Biopharmaceutical Drug Products for a Modern Age
Biopharmaceutical drugs are increasing in sophistication, requiring technological advancements to solve related challenges. The contributors to this BPI eBook highlight drug-product formulation concerns and collaborative efforts toward solving the fillâfinish conundrum. First, the BioPhorumâs Scott Ewan describes a holistic approach to containerâclosure integrity and the organization’s work toward developing and expanding upon that approach. Ewan explores how advancing analytical technologies, risk management, and quality by design (QbD) are changing the strategies related to containerâclosure integrity, which remains a significant aspect…
Are You Missing the Bigger Picture with Your AAV Analytics? Fast, Low-Volume Subvisible Particle Analysis for Characterizing Viral Vectors
Subvisible particle (SVP) analysis is a key indicator of stability and safety and is an essential parenteral drug quality metric. Yet assessment of SVPs is especially challenging in adenoassociated virus (AAV) vector formulations, in which limited precious sample is available. Traditional SVP methods consume large sample volumes, while dynamic light scattering (DLS), size-exclusion chromatography (SEC), and visual inspection can miss aggregates in the subvisible range entirely. This special report introduces backgrounded membrane imaging (BMI) technology as a solution for detection…
eBook: Biologics Stability â Lifecycle Management of Drug Products
The biomanufacturing industryâs increasing attention to risk mitigation through quality by design (QbD) and the emergence of complex therapeutic modalities have driven the need for a lifetime-management approach to assuring drug product stability. To that end, industry guidelines have been (or are being) developed to guide the industry toward a âholistic approachâ to conducting stability assessments. However, not all methods are stability indicating, and many more industry concerns need to be addressed. This BPI eBook offers perspectives on ICH Q12…
Formulation Development of Microbiome Therapeutics Localized in the Intestines
Scientists have discovered that microorganisms associated with the body play a critical role in human health (1). The intestinal microbiome is the collection of all combined genetic material of microorganisms in the intestines. An intestinal microbiota, a term frequently used interchangeably with microbiome, is the collection of such microorganisms in the intestine. With the volume of research into the human microbiome expanding, so too is the number of examples of its benefits to our health (1). Like diseases of the…
Introduction: Drug Product Discussions
Quality by design (QbD), risk management, and new technologies are shaping biologics formulation work in the 21st century. We saw much evidence of this at the BioProcess International Conference and Exhibition in Boston last fall, where a wide range of talks filled the Drug Product, FillâFinish, and Formulations track during the week after Labor Day. Dingjiang Liu (Regeneron) offered a high-level discussion from the BioPhorum Development Group (BPDG) on âAn Intercompany Perspective on Biopharmaceutical Product Robustness Studies.â Such studies ensure…
Analytical Strategies for Fixed-Dose Coformulated Protein Therapeutics
Coformulation of two or more proteins in a single formulation is an emerging approach to delivering multiple biotherapeutics that previously have been administered in sequence. This approach brings multiple benefits to all stakeholders. Foremost for patients, the primary benefits are combined therapeutic effects and improved convenience (e.g., fewer administration events). Healthcare providers see logistical benefits and decreased risk of medical errors. Additionally, coformulations also simplify manufacturing logistics, reduce costs of packaging and distribution, and provide new opportunities for product portfolio…
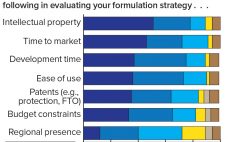
Early Stage Development of Advanced Formulations in the Drug Development Process Provides Competitive Advantages: Survey Predicts That Drug Product Formulation Recognition and Budgets Will Increase Significantly
New antibody formats and aggregate-prone, subcutaneously administered protein therapeutics present biopharmaceutical companies with major challenges regarding protein stability and aggregation. At the same time, protein stability often is not given enough attention in early stages of development. Protein aggregation reduces drug activity so that increasing doses are needed to achieve the same desired effect. Even worse, protein aggregates can induce immunogenecity that endangers patients and compromises product approval. A market study presented for the first time at the Bio-Europe 2018…