While the growth in biopharmaceutical manufacturing capacity in developed, major market countries is continuing its slow and steady climb, developing regions often are seeing double that growth rate. Over the past eight years, as detailed in the “About the Data” box, our company’s index of the top 1,000 biomanufacturing facilities (1) has tracked and ranked bioprocessing facilities worldwide in terms of known or estimated bioprocessing capacity (cumulative onsite bioreactor volume) number of biological products manufactured at clinical scale commercial scale…
Facility Design/Engineering
Designing Laboratories for Flexibility and Collaboration
The blockbuster business model may have paid off in the past, but tomorrow’s biopharmaceutical successes will depend more on rapid and diverse discovery than on any single breakthrough. In the race to get new therapies from research and development (R&D) into pharmacies, next-generation laboratory space could become a game-changer. Blockbuster drugs typically were made in industrial laboratories — and industrial-strength measures were required to reconfigure those spaces as new research priorities emerged over time. Facing changing patient needs and ongoing…
Capacity Strategies: The Strategies Behind Choosing Between Large-Scale and Single-Use Investments
Moderator Dan Stanton, with Weichang Zhou, Jenifer Wheat, Roger Lias, and Jim Vogel Single-use technologies (SUTs) are now prevalent within bioprocessing, but does this spell the end of industry’s historic reliance on stainless steel and fixed facilities? This roundtable was formed to discuss the wealth of investment in single-use (SU) equipment and flexible manufacturing solutions by contract development and manufacturing organizations (CDMOs) over the past few years, pitting that against what looks like a resurgence in fixed-cost stainless steel plants…
Facilities of the Future: Intelligent Design and Control Enable Quality, Efficiency, and Good Citizenship
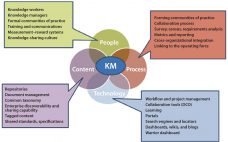
Today’s biomanufacturers need to be able to add capacity and capability quickly; provide increased supply service to customers on demand; and streamline the flows of personnel, traffic, utilities, and materials throughout bioprocess facilities. And companies need to be flexible enough to subtract capacity and retool quickly to produce new or different products. Many future facilities will be automated to some extent and use robotics in manufacturing. With personalized medicine on the rise, bioprocessors can benefit from colocation with academic research…
Flexibility, Automation, and Leadership: Drug-Sponsor Perspectives on Modern Biomanufacturing Facility Design
The title of this featured report — Smart(er) Facilities — came about in conversations with our KNect365 colleagues as they worked to plan this year’s BPI West conference, which took place 19–22 March 2018 in San Francisco, CA. For decades, biopharmaceutical facilities have incorporated cutting-edge designs for supporting processes, products, and human development. Each year, design innovations are rewarded for creating workspaces that facilitate both worker comfort and essential movement of promising drug candidates toward commercialization. In that context, biomanufacturing…
Partnerships for Progress: Supplier Perspectives on Facilities of the Future
Biomanufacturers are constantly tasked with making their products ever more efficiently with ever increasing quality. As major advancements come in biomanufacturing technology, companies find themselves in need of “smarter,” more flexible facilities than ever before. Recently, I asked several industry leaders for their thoughts on some criteria for smarter designs, including why there is such a demand: Cristina Amorim (vice president of facilities, EHS, and sustainability in the life sciences group of Thermo Fisher Scientific) Scott Battist (vice president, general…
Extend the Life of Your Facility: Flexibility Allows for Biopharmaceutical Process Innovation
For an industry built on constant change, there’s a surprising disconnect between the continuous drive for innovation and the inflexible facilities that house biopharmaceutical operations. Some of today’s facilities are built for today’s use with little thought about tomorrow’s. The typical approach for a new process or drug coming to market is to start with a brand-new building and permanently embedded equipment designed around that specific process. That approach is expensive and unsustainable. New bioproduction facilities can cost US$500 million…
Streamlined Column-Packing Design for a New Commercial Launch Facility
To meet network demand for a commercial launch facility, Genentech (Roche) designed a new downstream train and built it within an existing building shell at the company’s Oceanside, CA, site. This downstream train included new technologies to allow for rapid technology transfer of different new products in the company’s drug pipeline. One technology that was pursued was the Axichrom column platform from GE Healthcare and associated column packing equipment to streamline column packing design. Here we focus on how a…
BioPhorum Operations Group Technology Roadmapping, Part 3: Enabling Technologies and Capabilities
Although great strides have been made over the past 20 years to increase the productivity and robustness of manufacturing processes for biopharmaceuticals, the cost and complexity of their development and manufacturing remain high, especially in comparison with those of small-molecule pharmaceuticals. Process improvements are required to increase patient access while maintaining the viability of an R&D-driven biopharmaceutical industry. Facility productivity, cost of goods (CoG), and capital investment all have significant margins for improvement. Such goals can be achieved not only…
BioPhorum Operations Group Technology Roadmapping, Part 2: Efficiency, Modularity, and Flexibility As Hallmarks for Future Key Technologies
For a complex biopharmaceutical industry, setting out to forecast future technologies must involve considering how such technologies will be used. In the first article (1), I discussed why there was a need to develop a technology roadmap for the biopharmaceutical industry and the trends shaping its future: namely, the introduction of new product classes, the continued growth of the biopharmaceutical market, pressure to reduce costs, and uncertainty in approval and sales of new products. Herein I discuss the technology roadmap’s…