Manufacturing and facility challenges facing cell and gene therapy companies are similar to but more complex than those encountered by companies that produce traditional biopharmaceuticals such as vaccines, monoclonal antibodies, and other therapeutic proteins. A single product can have multiple components, manufacturing of which may or may not be outsourced. Project timelines are short, production technologies are new and evolving, and clinical demands change rapidly. Increasing competition for contract manufacturing services requires reserving capacity far in advance, which in most…
Facility Design/Engineering
Designing the Right Strategy for Digital Transformation: How a Pragmatic Approach to Digital Transformation Can Help Biomanufacturers Adapt to a Challenging Future
Although the biopharmaceutical industry has enjoyed explosive growth over the past three decades, it still faces an assortment of challenges. Those include growing portfolio complexities, increased demand volatility, stringent regulatory requirements, increased pricing pressures, and growing technological complexities, all leading to severe pressure on profit margins. To overcome such pressures, biopharmaceutical operations need to become more reliable and agile, and they must realize efficiency gains in both manufacturing and supply chains. Digital transformation offers strong value opportunities, including a potential…
Shared Clean-in-Place Systems: To Share or Not to Share?
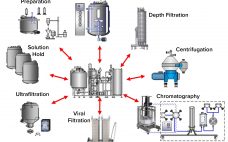
Risk of viral contamination is a an accepted part of developing biopharmaceutical products derived from mammalian-cell culture. Viral safety is achieved through a combination of complementary approaches such as selecting non–animal-derived raw materials, testing cell banks, testing for adventitious virus contamination during cultivation, and demonstrating viral reduction capacity of a purification process (1). The latter commonly is referred to as viral clearance by orthogonal purification. Clearly, viral clearance and appropriate viral segregation are important considerations in biopharmaceutical manufacturing process and…
Risk and Lifecycle Management for Biopharma Operations
By working with the best biopharmaceutical companies for over a decade, 4Tune Engineering (4TE) has built a portfolio of services that enables companies to address current-century challenges. The biotechnology industry needs to address advanced therapies and personalized medicines and deliver explicit patient outcomes. Biologics today fall into four categories: monoclonal antibodies (MAbs), biosimilars, advanced therapeutic medicinal products (ATMPs), and cell and gene therapies (CGTs). Consequently, we can ask whether our manufacturing science and technology (MSAT) approaches are up to the…
Bioprocess Intensification – Fast, Flexible, and Efficient Solutions
Propelled by single-use systems (SUSs), biopharmaceutical companies are approaching the ideal of continuous bioprocessing. In addition to improving process integrity and decreasing production costs, SUSs have enabled exciting ways to configure, operate, and evaluate manufacturing steps. Sensitive process analytical technologies (PATs) and discriminating data analysis platforms are supplementing those developments, helping process engineers and operators to study and modify workflows in unprecedented ways. The goal now is to intensify: to apply increasingly nuanced process knowledge and growing technological capability in…
Single-Use Technologies: Accelerating Bioprocess Design with Key Insights from the Experts
Companies turn more and more to single-use technologies (SUTs) to mitigate production challenges — and with good reason. SUTs clearly decrease conventional costs while increasing process integrity. Yet as the writers in this compilation suggest, SUTs are now making possible new, exciting ways to configure, operate, and evaluate biomanufacturing. In this compilation, BioProcess International gathers key insights from biopharmaceutical industry experts at Sartorius Stedim Biotech to explore how SUTs can realize high-quality yet cost-effective end-to-end bioprocessing. The studies herein identify…
Large-Scale Capacity Strategies: Single Use, Multiuse, or Both?
Early manufacturing facilities for large-scale production of biopharmaceuticals were, by necessity, very large. Low expression titers and blockbuster-market products such as monoclonal antibodies (MAbs) combined to require massive bioreactors — with capacities of 10,000– 20,000 L or more — and supporting infrastructure. In my early days covering the industry, I visited a few such facilities and was always awed by the huge tanks and what seemed like miles of piping. A 2010 Pharmaceutical Engineering article described a process modeling approach…
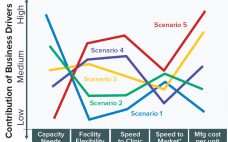
Biomanufacturing Scenarios: From the Biomanufacturing Technology Roadmap
Drug Substance Scenarios Given the complexity of the biopharmaceutical industry and the increasing diversity of products and companies, it is clear that there will be no “one size fits all” solution to biomanufacturing. Instead, we see a range of biomanufacturing scenarios playing out over the next 10 years. Five high-level scenarios were selected for drug substance manufacturing and two for drug product to cover the full spectrum of process and facility types. Each facility type is associated with a representative…
Opportunities for Modern Robotics in Biologics Manufacturing
It should come as no surprise to anyone familiar with biomanufacturing that current designs of bioprocess facilities as well as associated manufacturing spaces and support operations require excessive amounts of manual labor and manual interventions that lead to high labor costs and, consequently, total cost to supply. From receipt of raw materials to process execution and performance review, resolution of quality issues, and product shipping, no industry devotes a greater percentage of operating costs (or cost of goods sold, CoGS)…
Computational Science Changes Biolaboratory Design
Until relatively recently, life-science research was characterized by test tubes, Petri dishes, and centrifuges. Now, as with many industries, the life sciences are undergoing a digital transformation. Computational science is changing laboratory design. The healthcare industries always have generated large amounts of data. What has changed is the available information technology. With the growth of cloud computing, large data sets — and the high-speed tools for analyzing them — are available increasingly to a degree not possible with traditional servers…