Bio-Process Systems Alliance (BPSA) was formed in 2005 as an industry-led international industry association dedicated to encouraging and accelerating the adoption of single-use manufacturing technologies used in the production of biopharmaceuticals and vaccines. Corporate members include plastic equipment suppliers, service providers, and users in the biopharmaceutical industry who share this mission. A key focus of BPSA’s core activities is to educate its members and others through sharing of information and development of best practice guides that help suppliers, users, and…
Cell/Gene Therapies
The Role of Single-Use Polymeric Solutions in Enabling Cell and Gene Therapy Production –
Part 2: Regulatory Overview
The Bio-Process Systems Alliance (BPSA) was formed in 2005 as an industry-led international industry association dedicated to encouraging and accelerating the adoption of single-use manufacturing technologies used in the production of biopharmaceuticals and vaccines. Corporate members include plasticequipment suppliers, service providers, and users in the biopharmaceutical industry who share this mission. A key focus of BPSA’s core activities is to educate its members and others through sharing of information and development of best practice guides that help suppliers, users, and…
The Role of Single-Use Polymeric Solutions in Enabling Cell and Gene Therapy Production: Part 1: Introduction and Manufacturing Process
by Bio-Process Systems Alliance Cell and Gene Therapy Committee The Bio-Process Systems Alliance (BPSA) was formed in 2005 as an industry-led international industry association dedicated to encouraging and accelerating the adoption of single-use manufacturing technologies used in the production of biopharmaceuticals and vaccines. Corporate members include plastic-equipment suppliers, service providers, and users in the biopharmaceutical industry who share this mission. A key focus of BPSA’s core activities is to educate its members and others through sharing of information and development…
A Perspective on GMPs for Cellular Therapy Commercialization
Cellular therapies can be classified by therapeutic indication, by cell types, and by whether cells are taken from and administered to the same individual (autologous) or derived from healthy donors (allogeneic). Regulatory classification of cellular therapies differentiates among minimally manipulated cells for homologous use, transplants or transfusions, and cells that are more than minimally manipulated and regulated as medicines. Medical cellular therapies must meet quality, safety, and efficacy standards to obtain marketing authorization (1–8). Such therapies can be subdivided into…
Cell and Gene Therapy Data Management: Solutions to Address Complex Challenges
At the Phacilitate Leaders World and World Stem Cell Summit 2019, held 22–25 January, Steve Goodman (head of drug product manufacturing at bluebird bio) and Robert Di Scipio (CEO of Skyland Analytics) shared the podium to address what the product and process data-management ecosystem looks like for cell and gene therapy (CGT) development and manufacturing. A starting point for their presentation was that CGT development presents significant data challenges: capturing and analyzing product development and manufacturing data, tracking the collection,…
Is the QbD Toolbox Ready for Cell and Gene Therapies? Integrating Patient Outcomes into Manufacturing Cell and Gene Therapy Bioproducts
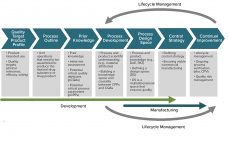
In their lifecycle development and manufacturing models, biotechnology products and the biopharmaceutical industry have been founded on principles originating from the pharmaceutical small-molecule industry. Such principles define clinical programs that establish risk benefits of a dosage and its delivery system on healthy individuals and patients. A company then develops a process to manufacture that product consistently over several years. Product quality attributes set through manufacturing controls are expected to ensure patient outcomes in terms of safety and efficacy and deliver…
Biopharmaceutical Characterization,
Part 1: Biological Assays —
A Conference Report
In late October 2018, KNect365 brought together more than 250 analytical specialists to discuss characterization of well-characterized biologics in Rockville, MD. Speakers from the US Food and Drug Administration joined experts from leading biopharmaceutical companies, service providers, and consultancies, including BPI editorial advisor Nadine Ritter (president and analytical advisor of Global Biotech Experts). She began the final day moderating a special town-hall session where audience members could pose their regulatory questions to a panel of FDA reviewers, and she ended…
Standardizing Human MSCs As Critical Raw Materials in Cell Therapy Products: Streamlining Clinical Translation
Advancements in cell therapy, biofabrication, and synthetic biology have driven the growth of the global regenerative medicine (RegenMed) industry in the past decade. The industry has developed innovative treatment options for patients with otherwise unmet medical needs (1). Human or animal cells or tissues are used as critical raw materials in cell therapy products that can replace, regenerate, or augment patients’ diseased, dysfunctional, or injured cells, tissues, or organs. These cells or tissues can be unmanipulated, or their biological characteristics…
The Need for Adherent Cell Manufacturing: Production Platform and Media Strategies Drive Cell Production Economics
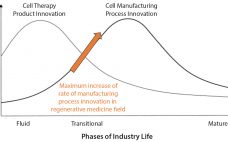
Most commercial biopharmaceuticals originated from academic research laboratories and start-up development laboratories. Despite such products having differences in modalities and targeted disease indications, and whether their target patient populations are relatively small or approaching blockbuster status, at a key point in development, biopharmaceutical production must scale up from laboratory to commercial production. That movement from research to development and then to manufacturing forces attention on economics and speed to market, and it drives innovative approaches to producing biopharmaceutical cell compositions…
Cell Culture Media: An Active Pharmaceutical Ingredient or Ancillary Material?
Cell-based therapies are used to treat diseases that require the replacement of diseased, dysfunctional, and injured cells (1). To produce these therapies, a wide range of reagents and materials such as antibodies, growth factors, and enzymes are used in their manufacturing processes. Such necessary materials are administered through a cell culture medium. Active pharmaceutical ingredients (APIs) are the main ingredients that make products therapeutic. Ancillary materials (AMs) and raw materials (RMs) are essential components used during production but are not…