The pace with which biosimilar drugs have been adopted in the United States has frustrated (and displeased) policymakers (1). After passage of the Biologics Price Competition and Innovation Act (BPCIA) (2) as part of the Affordable Care Act of 2010 (3), policymakers intended and expected significant reductions in expenditures for this class of biopharmaceuticals (4). The Federal Trade Commission (FTC) had predicted that the percentage of savings would be lower than that of the <90% reduction in costs for small-molecule…
Biosimilars
Biosimilars Pipeline and Market Trends
Most biopharmaceutical industry experts now consider biosimilars to be mainstream products, indicating that the field has progressed immensely over the past 10 years. Nevertheless, when comparing approvals and commercial offerings across the globe between 2013 and 2020, it becomes clear that some regions welcome these therapies more than others do. Western European biosimilars markets continue to be kind to these drugs’ production, distribution, and coverage; and companies headquartered in Asia and the Pacific Rim increasingly are getting involved in biosimilars…
A Healthy Biosimilars Market Promotes Innovation and Affordability
Innovative drug manufacturers require an opportunity to recoup capital and opportunity costs that they incurred to develop new medicines. Once patents have expired, competitors should be empowered to promote widespread affordability. An exclusivity period followed by a competitive market would promote the otherwise incompatible objectives of incenting innovation and promoting affordability. That careful balance exists for small-molecule medicines but not yet for biologics. The innovation side of the biologics market is working as intended, but a robust market for lower-cost…
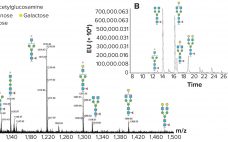
Building Orthogonality into Biosimilar Testing
Both the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) have developed regulatory guidelines on biosimilars (1, 2). These comprehensive documents provide clear guidance on what the agencies expect from structural characterization studies. Using state-of- the-art instrumentation and techniques is expected, but the use of orthogonal techniques in structural comparability assessments is also required. The application of orthogonal analytical techniques will provide a firm structural foundation to claims of biosimilarity. Characterization methods verifying and supporting conclusions drawn…
Evaluating Biosimilars: A View from the Small-Molecule World
For many years the pharmaceutical industry was dominated by small (usually synthetic) molecules, mixed with a number of nonactive materials and encapsulated or (in the really old days) rolled into pills or pressed into tablets. Although synthesizing the active pharmaceutical ingredients (APIs), formulating the dosage forms, and analyzing the materials at every stage of a product life cycle were not always trivial activities, they were relatively straightforward. Most of the tools needed for analyzing/controlling each step of the manufacturing process…
Risk and Lifecycle Management for Biopharma Operations
By working with the best biopharmaceutical companies for over a decade, 4Tune Engineering (4TE) has built a portfolio of services that enables companies to address current-century challenges. The biotechnology industry needs to address advanced therapies and personalized medicines and deliver explicit patient outcomes. Biologics today fall into four categories: monoclonal antibodies (MAbs), biosimilars, advanced therapeutic medicinal products (ATMPs), and cell and gene therapies (CGTs). Consequently, we can ask whether our manufacturing science and technology (MSAT) approaches are up to the…
Biosimilarity Assessments: The Totality of Evidence Framework
Biosimilars are evaluated through comparisons with their reference products using abbreviated pathways that have evolved significantly over the past few years. Scientists and regulators now accept that some quality attributes can vary from batch to batch over a product’s lifecycle, even for reference products. Moreover, reference and similar biotechnology products can show differences in noncritical quality attributes but still demonstrate comparable efficacy and safety (1). Here we describe a similarity assessment approach that is also applicable to comparability of lifecycle…
Biologic Labels and Induced Patent Infringement: A Perspective on Evolving US Law
The mechanism for proving patent infringement is changing for developers of both branded and follow-on biologics (either biosimilar or interchangeable). Here we examine how drug labeling can establish infringement, thus affecting follow-on manufacturers accused of inducing others to infringe patents on methods of treating medical conditions. Because precedent is paramount in the US legal system, judges look to small-molecule case history to help them understand alleged infringement by follow-on biologics. The classic approach to induced infringement of generic small-molecule drugs…
Biosimilars: Challenging the Justifications for Clinical Testing
The Biologics Price Competition and Innovation Act (BPIA) of 2009, describes the need for clinical trials as follows (1): “(cc) a clinical study or studies (including the assessment of immunogenicity and pharmacokinetics or pharmacodynamics) that are sufficient to demonstrate safety, purity, and potency in one or more appropriate conditions of use for which the reference product is licensed and intended to be used and for which licensure is sought for the biological product.” However, all the above studies are left…
Innovators and Biosimilar Companies: Experts Predict Intense Conflicts Ahead
CPhI Worldwide (organized by UBM) announced last fall the final section findings of the fifth edition of the CPhI Annual Report. Presented live at the meeting in Frankfurt, Germany, the report is now available online. It highlights immediate and long-term trends in pharmaceutical data, regulation, generics, and biosimilars. Four experts gave their views. They warned that the US Food and Drug Administration’s (FDA) approach to achieving six-sigma (nearly perfect or 99.9997% defect-free) quality is failing in respect to the pharmaceutical…