Through the acquisition of Bionova Scientific, the US arm of Asahi Kasei Medical will add a full-service biologics CDMO to its arsenal. US-based Bionova, which fully transitioned into a contract development and manufacturing organization (CDMO) in November 2021 with the opening of its $25 million Fremont, California facility says the acquisition adds a “strategic complementary addition” to Asahi Kasei’s bioprocess consumables, equipment, and biosafety testing services business. “We are extremely excited to become a part of Asahi Kasei. This represents…
Featured Content
eBook: Potency Bioassays — Development, Trending,
Transfer, and Automation
Bioassay development is a complex process that must be undertaken with great rigor and attention to detail. Potency testing experts use a range of methods including cell-based and binding assays. Consistency and reliability of results over time are paramount. Well-developed and -characterized methods are the end result of much phase-appropriate development work that goes on in parallel with bioprocess and biotherapeutic product development. This eBook begins with BPI senior technical editor Cheryl Scott’s report from the Biopharmaceutical Emerging Best Practices…
Establishing a Digital Platform for Data Science Applications in Biopharmaceutical Manufacturing
Biopharmaceutical manufacturing consists of multiple processes with complex unit operations. Those include mammalian cell culture in upstream operations and downstream chromatography steps for removing impurities from production streams and purifying the therapeutic biological molecule (1). Biomanufacturers need enhanced understanding to ensure the process control and manufacture of safe and efficacious drug products. Process understanding also enables opportunities for improving manufacturing efficiency. Both process understanding and optimization can be facilitated by leveraging large volumes of biotechnology data — typically generated during…
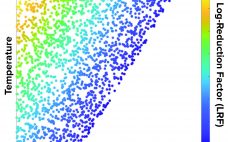
Pathogen Safety Digital Platform for Biopharmaceuticals: The Journey from Ground to Cloud
Digital transformation is at the heart of many biopharmaceutical companies’ strategies for ongoing success. However, the definition of digital transformation and what it consists of differ within the bioprocess industry (and might even vary within a single company), specifically as it applies to the overall value chain from R&D to clinical trials, manufacturing, supply chain, and eventually commercial operations. To provide a perspective on what digital transformation could look like in bioprocessing, we present a case study about an exploratory…
Get High-Throughput, Definitive Identification of Viable Cells and More in Your Cell Therapy
Analyzing for viable cells using traditional methods such as flow cytometry often encounters clogging resulting in the loss of precious cell therapy samples. Not only is it low throughput, but incredibly complicated, which can lead researchers to misidentify cellular and non-cellular material and confuse cell viability results with product-purity issues. Additionally, it is a regulatory requirement for all injectable drug products be characterized for sub visible particles (SVP) and aggregates that may form during a manufacturing process. With Backgrounded Membrane…
Addressing Critical Issues in Cell and Gene Therapy Manufacturing
Recombinant lentivirus (LV) and adeno-associated virus (AAV) are critical components of cell and gene therapies, which show great promise for treatment of diseases from genetic disorders to cancer. Accordingly, there is an unprecedented need for high titer and large-scale viral vector manufacturing processes to support the growing number of researchers developing biotherapeutics for immunotherapy. Download this Special Report, highlighting: Evolutions of gene therapy manufacturing Improvements to GMP raw-material sourcing. Role of transfection reagents like VirusGEN® GMP as a turnkey element…
Patent Thickets Constrain US Biosimilars Market
Biosimilars represent a significant cost-savings opportunity in the United States because they can introduce competition for some of the most expensive and widely used prescription drugs on the market: originator biologics. Several market and regulatory barriers have slowed biosimilar market entry and uptake in the United States (1). Some hurdles are unintentional or transitory; others are deliberately crafted by manufacturers of reference biologics to thwart competition. Among the most significant strategic barriers to biosimilars’ entry into the US market are…
Reducing Cell and Gene Therapy Development Time and Cost with New Purification Strategies
The past 40 years have ushered in the most advanced medicines the world has ever seen, with tremendous improvements in biomanufacturing technologies to enable their development. Advances in production technology have brought significant improvements in upstream productivity, which then caused bottlenecks in downstream processing. Although many bottlenecks have been resolved for most biologics, new modalities such as gene therapies and mRNA vaccines are driving the need for differentiated purification solutions. Meanwhile, pressures to increase efficiency and reduce costs continue to…