With new customers on board and a significant backlog, Avid Bioservices says it is making incremental expansions in its network ahead of a major investment. Contract development and manufacturing organization (CDMO) Avid Bioservices presented its Q1 FY2020 financials this week. Revenues stood at $15.3 million (‚ā¨13.9 million) for the quarter, up 21% year-on-year, while revenue backlog increased 34% to $61 million. ‚ÄúThe highlights for the first quarter of 2020 were new customer contracts, significant backlog growth and another successful process…

Facilities & Capacity
FDA rejects Mylan and Biocon’s insulin glargine again
The US FDA has issued a second complete response letter for a follow-on insulin glargine product referencing Lantus made by Biocon at its facility in Malaysia. In June 2018, the US Food and Drug Administration (FDA) issued a complete response letter (CRL) to Mylan and its manufacturing partner Biocon rejecting their insulin glargine product submitted through the 505(b)(2) regulatory pathway as a follow-on biologic referencing Sanofi‚Äôs Lantus. Just over a year later and the firms have reported a second CRL…
Celltrion turns to CDMO Lonza for infliximab biosimilar
Supply of drug substance from Lonza‚Äôs stainless-steel facility in Singapore will complement production of Remsima (infliximab) within its own network, Celltrion says. Remsima is Celltrion‚Äôs biosimilar of J&J‚Äôs autoimmune best-seller Remicade (infliximab), approved by the EMA in 2013 and by the US FDA in 2016. It became the first biosimilar monoclonal antibody to be launched in Europe in 2015 and according to the Korean drugmaker it captured over 50% of the EU infliximab market by the third quarter 2018. While…
Not all about that base: Catalent organic downside offset by M&A
Biologics now represent 32% of Catalent‚Äôs revenues, but unfavorable product mix, plant maintenance and a customer moving drug substance in-house hit the CDMO‚Äôs pre-existing business in Q4 FY2019. For the fourth quarter fiscal year 2019, Catalent reported revenue of $726 million (‚ā¨662 million) across all its business units. While the contract development and manufacturing organization (CDMO) saw an 18% increase in sales year-on-year to $231 million within its Biologics and Specialty Drug Delivery segment, removing revenues from the recently acquired…
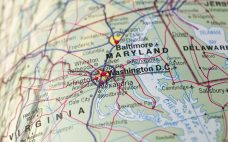
IBBR to lease biomanufacturing plant in ‚Äėhigh-demand‚Äô Maryland
The Institute for Bioscience and Biotechnology Research (IBBR) has invited biomanufacturers looking for a pilot plant within Maryland‚Äôs biotech corridor to apply to lease a former training plant. The 9,100 square-foot plant located just northwest of Washington DC in Maryland has been used by the IBBR to carry out GMP training and education programs but is now being offered to biotech and biomanufacturers by the institute, which is a collaboration between the University of Maryland and NIST. According to Viqar…
Think your CMO is high maintenance? Let’s hope so
Having a well-managed facility maintenance schedule can avoid lengthy shutdowns and lost revenues, says a regulatory expert. During the Q2 2019 reporting season, Samsung BioLogics reported revenues of KRW 78.1 billion ($65 million) compared to KRQ 15.1 billion recorded in the previous quarter. The Korean contract manufacturing organization (CMO) attributed the 38% drop to the plant operation rate temporarily decreasing at its 152,000 L plant number 2 during the quarter due to ‚Äúregular maintenance essential for biopharmaceutical manufacturing.‚ÄĚ According to…
Halix completes Dutch viral and protein product plant
Operations at the facility will begin later this year to feed the demand for proteins and viral vectors from small to midsize biotechs says CDMO Halix. Construction on the 6,700 m2¬†plant began a year ago and this month the contract development and manufacturing organization (CDMO) announced it is ready to produce biopharmaceutical drug substances. The facility, located in the Leiden Bio Science Park, The Netherlands, boasts a viral vaccine and viral vector manufacturing line along with a separate a separate…
Pfizer pumps $500m into NC gene therapy site
The expansion in North Carolina will create 300 jobs and is the latest investment by Pfizer in its burgeoning gene therapy network. The $500 million (‚ā¨450 million) investment in Sanford, North Carolina will see the Big Pharma firm construct a facility to support production of its recombinant adeno-associated virus (rAAV) vectors for use in its gene therapies and vaccines. Mike McDermott, president of Pfizer Global Supply, said the Sanford expansion will ‚Äústrengthen Pfizer‚Äôs leadership in gene therapy manufacturing technology‚ÄĚ and…
Commercial N-1 perfusion carried out by Samsung BioLogics
CDMO Samsung BioLogics has used an Alternating Tangential Flow (ATF) system to perform N-1 perfusion at its 180,000 L facility in South Korea. Contract development and manufacturing organization (CDMO) Samsung BioLogics opened its number 3 facility at its site in Songdo, Incheon last year, boasting 180,000 L of commercial stainless steel bioreactor capacity. This month, the firm has announced it has successfully carried out N-1 perfusion with an Alternating Tangential Flow (ATF) system at the 3,000 L scale to supply…
Evotec eyes US commercial biomanufacturing pod
Months after buying platform firm Just.Bio, Evotec says it is evaluating the construction of the first modular J.POD for commercial manufacturing later this year. In May, German R&D services firm Evotec launched itself into the large molecule space through the $90 million (‚ā¨81 million) acquisition of Just.Bio. The deal added several platform technologies aimed at reducing the cost of goods for proteins, including a large molecule manufacturing design platform, known as J.DESIGN and JP3, a lab and computational tool for…