Triton X-100 detergent makes an interesting case study in bioprocess sustainability strategy. Also known as octylphenol ethoxylate (OPE), this nonionic surfactant has many uses in biopharmaceutical research and development. Among other laboratory applications, it is used to lyse cells and DNA in research, to solubilize membrane proteins and decellularize animal-derived tissues, to reduce the surface tension of aqueous solutions during immunostaining, and to remove sodium dodecyl sulfate (SDS) from polyacrylamide gel electrophoresis (PAGE) gels for analysis. It also serves as…
Downstream Processing
Viral Risk Mitigation: A Global Regulatory Perspective
The production of biologics will always have the risk of viral contamination. Manufacturers have developed a multitiered approach — tailored to individual processes — to prevent adventitious viruses from entering production processes, detect contamination in raw materials and process intermediates, and remove viruses in downstream purification. This article provides an overview of the global regulatory framework to ensure the viral safety of biologics. Past Contamination Events Past contamination events have resulted in corrective and preventative actions to reduce the risk…
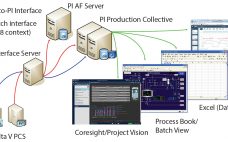
Using Data and Advanced Analytics to Improve Chromatography and Batch Comparisons
With all the hype surrounding the industrial Internet of Things (IoT), cloud computing, and digital transformations, the most important information technology factors still are data and the connections of sensors, systems, and applications that generate, store, find, and use those data to obtain operational intelligence. Data volumes are increasing rapidly, and they will continue to do so. The ability to find and make sense of data to obtain intelligence that improves process outcomes is more important than ever. For clinical-…
Intensification of Influenza Virus Purification: From Clarified Harvest to Formulated Product in a Single Shift
Influenza is a global respiratory disease with an estimated mortality of up to a half million people per year (1). The majority of traditional influenza vaccines are still produced in eggs. Downstream processing typically consists of clarification by centrifugation, concentration by ultrafiltration, and purification by ultracentrifugation (2). Recombinant vaccines are most often purified by chromatography. Chromatographic purification of viruses already has achieved major improvements in recovery and scalability (3), but it also is important because it enables virus purification to…
Sticking In or Standing Out? Dichotomy in Vaccine Purification By Chromatography
A general vaccine purification strategy can be divided into three stages, with one or more steps for each stage. The first stage is to concentrate and isolate the target molecule quickly to remove it from conditions that could lead to its inactivation or loss. Intermediate purification seeks to remove remaining contaminants, typically using an orthogonal approach. That is followed by a polishing step in which trace impurities are removed through high-efficiency steps because those impurities usually are similar to the…
Process Analytics and Intermediate Purification of Bispecific Antibodies with a Non-Affinity Platform
The therapeutic benefits of monoclonal antibodies (MAbs) have been demonstrated in recent decades with uncontestable success as treatments for human disease. Despite MAbs’ key features such as specificity, selectivity, and safety, the format has limitations (1, 2). Bispecific antibodies may overcome number of difficulties (3). Multiple formats of bispecific antibodies have been developed, although only the κλ-body is fully human and devoid of linkers or mutations. It requires no genetic modifications of heavy and light chains and results in bispecific antibodies…
Virus Segregation During Purification Processes: Calculation of Critical Potential Carryover of Viruses
Before a pharmaceutical product is introduced into humans, either in a clinical trial or as a marketed product, virus safety must be evaluated carefully. Virus safety normally is ensured using a three step complementary approach: selecting and testing cell lines and/or raw materials for the absence of viruses, testing the product at appropriate steps of production, and assessing the capacity of a production process to clear infectious viruses (1). The latter (also referred to as viral clearance) is the subject herein. Spiking studies are conducted to evaluate the capacity of a purification…
A UF–DF Screening System for Bioprocess Development: Efficient and Cost-Effective Process Fit and Scale-Up to Manufacturing
Ultrafiltration and diafiltration (UF–DF) of therapeutic proteins are performed in either tangential or crossflow mode using membrane filters. UF–DF plays a critical role in both downstream and upstream processes for the biopharmaceutical industry (1). In upstream production processes, classical tangential-flow filtration (TFF) or alternating tangential-flow (ATF) systems are used in high–cell-density perfusion for protein expression by cell culture (2). TFF is used in downstream processing for UF–DF and concentration of therapeutic proteins. TFF unit operations are common in protein purification…
In-Line Turbidity Sensors for Monitoring Process Streams in Continuous Countercurrent Tangential Chromatography (CCTC)
A strong connection between turbidity and total suspended solids (TSS) has been linked in the past to measuring well defined particles in processes. Optical density probes have seen wide adoption in the biotechnology industry for monitoring cell growth within a bioreactor, whereas in-line turbidity sensors have been used to monitor filter performance. Turbidity measurements offer a rapid quantification of suspended solids but have not been used in the biotechnology industry for chromatographic resins. In this study, turbidity measured with equipment developed by PendoTECH was used with novel continuous chromatography technology developed by Chromatan…
Dual Sourcing of Protein A Resin to Mitigate Supply Chain Risk: A Comparative Study to Determine Equivalence
Protein A affinity chromatography is a well-established technology that is used extensively for large-scale purification of monoclonal antibodies (MAbs). With this mode of chromatography, very high product purity can be achieved in a single, relatively simple unit operation. A solution containing the target protein of interest is applied to a liquid-chromatography column at near-neutral pH, and one or more wash steps follow to lower product- and process-related impurities (1). Product is eluted through application of a low-pH buffer. Finally, the…