The biopharmaceutical industry uses living biological systems as a platform for manufacturing of protein-based drugs, vaccines, and other therapies derived from or consisting of different cell types. On one hand, living systems are inherently susceptible to viral infection and may harbor endogenous viruses, so the potential for such contamination cannot be eliminated. On the other hand, the industry has an excellent patient-safety record. Viral safety is achieved through three fundamental measures: prevention (e.g., by selection), removal (by clearance and/or reduction),…
Downstream Processing
Detection and Clearance of Viruses in the Biopharmaceutical Industry
Viral contamination is a common threat to all animal- and human-derived biopharmaceuticals. This type of contamination can affect any part of a bioproduction process, so biomanufacturers need to perform viral testing studies and incorporate viral clearance methods into their processes. Viral contaminants can come from cell lines (e.g., endogenous retroviruses) or from adventitious (e.g., mycoplasma) introduction during drug manufacturing. Virus testing of master cell banks (MCBs), working cell banks (WCBs), end-of-production cell banks, and bulk unprocessed harvest material is called…
Monoclonal Antibody Aggregate Polish and Viral Clearance Using Hydrophobic-Interaction Chromatography
Hydrophobic Interaction chromatography (HIC) is a powerful polishing tool for the downstream purification and manufacture of biotherapeutics. HIC offers orthogonal selectivity for the clearance of difficult process and product-related impurities such as aggregates, host cell proteins and endogenous and adventitious viruses. In this study, a family of POROS HIC resins with novel ethyl and benzyl chemistries was used to successfully polish two clinical stage monoclonal antibodies harboring very high levels of product aggregation (>10%). In addition to aggregate removal, viral…
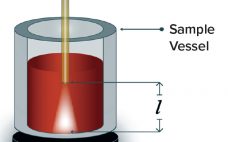
Viral Nanofilter Integrity: Using Variable-Pathlength UV-Vis Spectroscopy for the Gold Nanoparticle Test
Viral filtration (VF) using nanofilters removes endogenous and/or adventitious viruses from biologic drug-substance manufacturing processes (1). The gold particle test (GPT) is performed as part of postuse integrity testing — to complement postuse leakage testing — for cellulose filters such as Planova 20N filters from Asahi Kasei Corporation. First, a proprietary gold-colloid solution matched to the filter type (e.g., 20N) is filtered through the test article. That filter’s pore-size distribution can be assessed using spectrophotometric absorbance readings of the integrity-test…
Adenovirus Downstream Process Intensification: Implementation of a Membrane Adsorber
Historically, companies developing vaccines have used attenuated pathogens, inactivated infectious agents, or antigenic constituents purified from pathogenic sources. In the past 20 years, technological advances such as recombination and viral vectors, have enabled development of vaccines against diseases with previously no available treatments (1). Viral vectors have become one of the most rapidly evolving and promising fields in vaccinology and regenerative medicine. In addition to preventing infectious disease, they have a broad range of potential applications, including treatment of hereditary…
Addressing Regulatory Requirements for Filter Integrity Testing
Filter integrity is a fundamental element of sterility assurance during production of biopharmaceutical and vaccine products. Integrity test results are a key foundation for drug lot release, so any external element that could affect their reliability must be viewed as a critical issue. But when should a filter integrity test be performed? This article highlights the Sartocheck 5 Plus filter integrity tester as a means to address regulatory requirements. Please fill out the form below to read the full article…
Rapid Mammalian Cell Harvest Without Centrifugation for Antibody Purification: Using a Novel System for Cell Culture Media Clarification
Monoclonal antibody (MAb) expression systems typically use signal peptides to ensure secretion of antibodies into cell culture media. Although that reduces the complexity of purification and prevents the need for cell disruption, it does require using expensive and time-consuming techniques to separate cells from antibody-containing cell culture fluids. In this study, we describe our tests of the novel Sartoclear Dynamics Lab V system (Sartorius S Lab Instruments GmbH and Co. KG) for rapid clarification of cell culture media without requiring…
Control of Protein A Column Loading During Continuous Antibody Production: A Technology Overview of Real-Time Titer Measurement Methods
During production of therapeutic antibodies, harvest titer is measured to monitor product mass loaded onto the protein A capture column. This prevents both column underloading (underusing expensive resin) and overloading (wasting product as flow-through (FT)) while allowing for column yield calculations. Batch production yields a single homogenous harvest pool, thus only one titer measurement (along with volume loaded) is sufficient to determine the mass loaded. During continuous production, however, cell-free harvest (permeate) continuously exits a perfusion reactor and loads a…
A Shift of Mindset: How Single-Use Systems Influence Bioprocess Engineering and Project Execution
In past decades, the focus in bioprocess engineering was on traditional stainless steel project management, which is highly dependent on established project schedules. Many milestones, tasks, and deliverables during basic and detailed design and execution were implemented in the same tried and tested way by engineering, suppliers, and biopharmaceutical companies. Making decisions was complicated by alignment with long lead items. With the introduction of single-use (SU) technologies, the transition in execution and optimization of project management only just has been…
An Integrated Bioprocess for Antibodies: From Harvest to Purified Bulk in Six Hours
Antibody production platform processes have been widely adopted in biomanufacturing, but many unit operations are not suitable for integration and automation. Here we describe the work of integrating unit operations by transforming a column operation to a more robust cassette format. We have selected a biomolecule-friendly buffer (phosphate) to eliminate, or delay, the performance of a circulating tangential flow ultrafiltration/diafiltration (UF/DF) operation, so the harvest-to-purified-bulk process can be integrated, resulting in a single, direct-flow operation, that reduces the batch process…