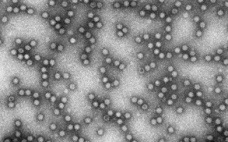
Until recently, downstream process development (PD) teams have lacked methods for easy, effective, and economical estimation of a process’s viral clearance capability. David Cetlin (senior director of R&D at Cygnus Technologies) delivered an “Ask the Expert” presentation on 13 October 2020 describing how MockV kits containing noninfectious mock-virus particles (MVPs) could fill that gap. Cetlin’s Presentation Viral clearance studies tend to be outsourced to contract research organizations (CROs) because they require biosafety level (BSL)-2 and -3 facilities for working with…
Downstream Processing
Exploring Resin Modalities for Biotherapeutic Purification
New chromatography supports must demonstrate improved selectivity, and bead technologies must be optimized for high binding capacity and product recovery. Drug manufacturers also need access to expertise and continued support from chromatography suppliers that can assist with method development and design of experiments (DoE) assessments. Working together, these industry groups can accelerate method development, increase process yield, reduce buffer consumption, minimize the number of unit operations, and improve overall process economies. Learn more in this Special Report about how resin…
eBook: Downstream Processing — An Overview of MAb Purification Methods
The downstream harvest, clarification, and purification operations of biologics are essential steps to ensure drug product safety. However, these process steps can be problematic for complex biologics. Compared with processes for traditional small-molecule pharmaceuticals, downstream methods for monoclonal antibodies (MAbs) have higher risks of contamination. Thus, different centrifugation, filtration, chromatography technology and viral clearance/inactivation strategies must be applied to remove dead cells, host-cell proteins, viruses, and other contaminants. Several factors must be considered to determine which methods and technologies are…
Drug Formulations Are Changing:
New Sterile Filtration Challenges in the Changing Landscape of Drug Formulations
Read about the challenges of sterile filtration of high concentration mAbs, liposomes, and lentiviral vectors, and how to solve them in this Special Report. Development of new, complex drug formulations has given us therapeutics with properties that are markedly different from traditional drug types. High viscosity or low surface tension formulations or large viral vector molecules can mean that sterile filtration processes, which are optimized for traditional drug types, are not as efficient for the new, complex formulations. Premature filter…
Development of a Single-Use Hermetic Centrifuge System for Mammalian Harvest with Moderate to High Cell Content
The production of increasingly higher cell densities has stressed the already limited solids-handling capabilities for traditional intermittent ejection centrifuge systems. By contrast, a single-use disc-stack centrifuge based on the solids-flow principle offers distinct advantages for cell culture harvesting. Such benefits include solids handling of high-density cell culture processes and elimination of the separation disruption and aerosol generation associated with the intermittent solids ejection. A single-use system also provides well-established benefits of disposable components — such as removal of steam- and…
A New Runway for Purification of Messenger RNA
A high-performing capture method is a critical bedrock asset for developing industrial purification processes. This is especially true for extended families of products that share highly similar chemical composition. Therapeutic monoclonal IgG is an example. The ability of protein A affinity chromatography to achieve 95% purity in one simple step was the runway that got recombinant immunotherapy off the ground and made it available to millions. In fact, protein A did more. Beyond giving the industry a foundation manufacturing method,…
How Much Harm Can a Single Droplet Do? Considerations for a Viral Inactivation Step
Viral clearance is a fundamental aspect of viral safety for biopharmaceutical products. Regulatory agencies around the world require biomanufacturers to segregate their operations appropriately to mitigate the risks of carryover contamination from previous process steps or product batches and of crossover contamination between product(s) made in the same facility. Guidelines are vague in defining “appropriate,” leaving biomanufacturers to interpret regulatory expectations and define their own virus reduction and segregation strategies. Given the differences among manufacturing processes and facilities housing such…
Dye-Stripping Buffer and Resin Stripped-Dye Analysis: Development and Optimization of a Novel Spectrophotometric Assay and Method for Removal of Cibacron Blue Dye
Biopharmaceutical process-related impurities encompass all organic and inorganic materials that arise from the biomanufacturing process apart from the drug substance. According to ICH Q6B guidelines on test procedures and acceptance criteria for biotechnological products, these impurities include cell substrates such as host-cell proteins, host-cell DNA, endotoxins, and so on; inducers, antibiotics, media components, and chromatographic media used in purification processes; and solvents and buffer components (1). Impurities can cause undesired, deleterious pharmacological effects if ingested or injected by a patient,…
Streamlined Polishing and Viral Clearance Using a New Hybrid, Biomimetic, Single-Use Anion Exchanger
Flow-through anion-exchange (AEX) chromatography is used frequently in biopharmaceutical purification processes for reduction of net–negatively charged host-cell proteins (HCPs) and viruses as part of a validated viral clearance strategy (1, 2). AEX column chromatography is the technology most often used for electrostatic viral clearance, particularly in commercial-scale biopharmaceutical manufacturing, for which columns have a long-established history of reliable and well-understood performance (3). Still, validation of HCP and viral clearance by AEX columns in biopharmaceutical processes involves complexities that contribute significantly…
Simple and Effective Method for Purification of DMT-On Oligonucleotides Using HIC Resins
Within the biopharmaceutical industry, oligonucleotide drug pipelines have increased significantly because of the effectiveness of such drug products in treating devastating diseases. Downstream specialists need improved purification techniques for such highly valuable materials. Dimethoxytrityl (DMT) is used to synthesize oligonucleotides and temporarily mask the characteristic chemistry of the 5Í´-hydroxy functional group. DMT can be left on an oligonucleotide following synthesis to provide stability to a molecule during subsequent processing. Herein, we describe a novel, effective, and high-recovery method for purifying…