Interest in industrial-scale production of messenger RNA (mRNA) has surged amid rapid development of mRNA-based vaccines against SARS-CoV-2. During an 18 February 2021 Ask the Expert presentation, Aleš Ĺ trancar (chief executive officer of BIA Separations, a Sartorius company) reminded attendees that no platform approach yet exists for mRNA production and that much remains to be learned about manufacturing such products at commercial scales. He described current production challenges and shared BIA’s efforts to devise flexible mRNA purification tools. Ĺ trancar’s Presentation…
Downstream Processing
eBook: Buffers — Navigating New Demands on Downstream Raw Materials
Bioreactor titers for monoclonal antibody (MAb) processes have increased significantly since the dawn of the biopharmaceutical industry, yet such gains have instigated bottlenecks for critical high-volume raw materials used in downstream processing, such as buffer solutions. As downstream purification is required for most, if not all, biopharmaceutical products, buffers and their preparation are topics that concern nearly every drug company. But those topics rarely receive direct attention. This BPI eBook explores what factors prompted the current buffer bottleneck and what…
How to Improve the Capturing of Antibody Fragments
Some of the latest promising biopharmaceutical drug substances are antibody fragments. Antibody fragments are either separate functional subunits of antibodies or recombinant molecules, which, just like antibodies, are composed of immunoglobulin domains. These drugs offer several therapeutic advantages over conventional monoclonal antibodies. Upstream processing for antibody fragments is easier than it is for standard antibodies. Recombinant-based antibody fragments can be modified to meet specific needs of affinity, avidity, valence, and action mode. They also can be produced in prokaryotic cells…
Customized Yeast HCP Quantification with Biolayer Interferometry Using a Horseradish Peroxidase Substrate
Biopharmaceuticals are the largest group of drugs under development (1), and the demand for new and safe drug products is high. The most common bacterial and mammalian cell lines for production are Escherichia coli, Chinese hamster ovary (CHO) cells, and yeast. During a production bioprocess, a cell line expresses not only the molecule of interest, but also host-cell proteins (HCPs). They are considered to be impurities in a final drug product because they can affect the efficacy and safety of…
Ask the Expert: FPLC Column Selection Considerations
On 10 November 2020, BPI presented an “Ask the Expert” webinar with Dan Yukon (head of North American and global SNAP product sales at Astrea Bioseparations) on considerations for selecting analytical fast-protein liquid chromatography (FPLC) columns. With many options on the market, deciding which type and brand to use can be difficult. To help take out the guesswork, Yukon addressed a number of topics, including pressure and volume considerations; column configuration; materials of construction; frit type, design porosity, and mounting;…
Ask the Expert: Centrifugation Guided By Optical Sensors Enables Efficient, Reagent-Free Cell Separation
Ben Josey, PhD (field application scientist at Corning Life Sciences), joined BPI on 3 December 2020 to deliver an “Ask the Expert” presentation about using optical-sensor–guided centrifugation for cell therapy development. Cell-separation techniques fall into four basic categories: filtration, centrifugation, affinity purification, and emerging methods such as microfluidics and acoustofluidics. Selecting the technology best suited to an application requires careful balancing of method precision and process efficiency, the latter of which includes factors such as batch size, time, labor, and…
Removing Oligomers of a Recombinant Human Therapeutic Hormone:
Evaluation of Chromatographic Options for Effectiveness
Aggregation is a common cause of protein instability, which renders a biologic product unfit for therapeutic use. Sometimes it is difficult to purify monomeric proteins from oligomers because of similarities in their isoelectric points (pIs). Proteins such as hormones have pI ranges similar to their oligomers and thus can be difficult to separate out using a conventional polishing chromatographic step such as ion exchange. With those pI similarities, removal of oligomers to a considerable extent by ion exchangers can compromise…
Evaluation of a Novel Peptide-Based Affinity Ligand for Human IgM Purification: Use of an Automated Liquid-Handling System for Rapid Assessment of Binding Kinetics and Capacity
One-step affinity purification of antibodies is a powerful and widely used tool in the biopharmaceutical industry. Although different strategies can be used to purify immunoglobulin isotype G (IgG), the larger antibody isotype IgM has limited options. Human IgM is the largest antibody and primarily exists as a pentamer in the bloodstream (1). IgM molecules exhibit higher avidity than other antibodies do and serve as essential activators for the complement cascade (1–3). Approaches to IgM purification with hydroxyapatite and ion-exchange (IEX)…
eBook: Ion-Exchange Chromatography for Modern Biopharmaceutical Purification
Understanding the functionalities of chromatography resins can improve the product yield and purity in a biotherapeutic purification workflow. Ion-exchange (IEX) chromatography separates biomolecules on the basis of charge. For several reasons, it is the most widely used separation tool for purification of biopharmaceutical products. IEX is a well-characterized purification method with high binding capacity and flexible selectivity. It also works with mild operating conditions that help to preserve the biological activity of a biopharmaceutical drug substance. That versatility enables several…
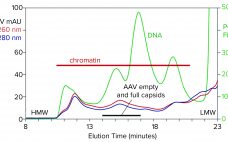
Streamlining Industrial Purification of Adeno-Associated Virus
With its first licensed therapeutic now marketed worldwide (1), adeno-associated virus (AAV) has become a preferred vector for gene therapy. However, unlocking its full potential still poses challenges, many of which are associated with purification. The first involves the transition from upstream to downstream processes. AAV-bearing lysates are laden with debris that foul filtration media and limit or prevent concentration. Another challenge involves reduction of soluble host-cell DNA, which is complicated by its strong association with nucleoproteins. A third involves…