Disposable materials have been used in many aspects of biomanufacturing since muromonab was first launched in 1986. Single-use stirred-tank bioreactors first became commercially available from HyClone in 2004 (1). Despite their demonstrated value to bioprocessing, disposable materials remain the subject of wide-ranging differences of opinion. Discussions of any technology are healthy and important for identifying areas for improvement, but some hearsay and bold propositions made regarding single-use components and the environment are not always helpful. Sustainability is an important and…
Downstream Processing
eBook: Development and Application of a Simple and One-Point Multiparameter Technique — Monitoring Commercial-Scale Chromatography Process Performance
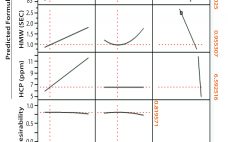
In commercial-scale biopharmaceutical manufacturing, downstream chromatography steps are still a bottleneck and contribute to significant operational costs (1, 2). Some of those costs are inherent (e.g., resins, large buffer quantities, and cleaning) whereas others are avoidable (e.g., product loss due to rejected lots or deviations that result in production downtime). Maintaining efficient and robust chromatography process performance is therefore critical for minimizing operating costs. To do so, we introduce a simple and one-point multiparameter technique (SOP-MPT) for monitoring chromatographic process…
The Multi-Mode Mimetic Ligand Library: A New Tool for Rapid Development of Downstream Processes
Recent developments in downstream processing of biomolecules — including continuous processing, bind–elute affinity capture, and flow-through polishing steps — have increased the need for greater selectivity from chromatography adsorbents. This has led to the introduction of a new generation of adsorbents: so-called “mixed-mode” or multimodal ligands. They provide greater selectivity and tolerance to process buffer composition than either ionexchange chromatography (IEC) or hydrophobic-interaction chromatography (HIC) alone can provide. Learn more in this Special Report from Steve Burton, Chief Executive Officer…
Scale-Up of Twin-Column Periodic Countercurrent Chromatography for MAb Purification
Periodic countercurrent (PCC) processes increasingly are being evaluated as alternatives to single-column batch capture processes. Some of the advantages of PCC processes over single-column processes include shortening of processing time and/or reduction of required resin volume through increased productivity; reduction in resin costs through improved resin capacity use; and reduction in buffer consumption through increased column loading. Those advantages, however, come with increased equipment complexity and hardware costs. PCC processes and systems with two to up 16 columns of the…
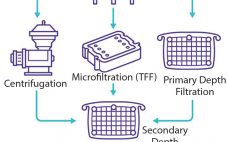
Continuous Solids-Discharging Centrifugation: A Solution to the Challenges of Clarifying High–Cell-Density Mammalian Cell Cultures
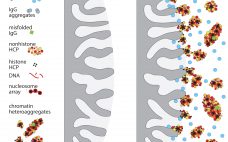
Clarification is typically the first unit operation in the purification of monoclonal antibodies (MAbs) and other proteins from mammalian cell cultures. This process removes cells and cellular debris from the culture fluid to produce a clarified cell culture supernatant that will be suitable for further purification (1). Before 2000, depth and tangential-flow microfiltration were standard clarification technologies in the biopharmaceutical industry. Process-scale centrifugation was considered to be a significant capital investment, and bioprocessors had limited ability to control (minimizing) the…
Filter-Based Clarification of Viral Vaccines and Vectors
Viral vaccines rely on the antigen properties of a virus or virus-like entity to trigger an immune response and induce immune protection against a forthcoming viral infection. Through development of recombinant viral vaccines, developers can reduce risks associated with the presence of live and inactivated viruses. Instead, recombinant vaccines induce immunity against a pathogen by relying on the capacity of one or more antigens delivered by means of viral vectors or the baculovirus/plasmid system (1). Viral vaccines are formulated with…
A Two-Step Purification Process: Application of HIC Membrane Chromatography in a Disposable 2,000-L Clinical Facility
Given paradigm shifts in the biopharmaceutical industry over the past decade, product development timelines are squeezed as the number of molecules entering clinical development continues to increase. Manufacturing facilities, especially those supplying clinical trial materials, have had to adapt to this trend. One popular approach is to have fully disposable equipment that allows for quick product changeover and flexible manufacturing capacity to respond to variable clinical demand. Although many facilities-related technologies exist to support that concept (e.g., disposable probes and…
IgG Purification By Ultrafiltration: Time for Another Look
One of the early disappointments in development of immunoglobulin G (IgG) purification technology was ultrafiltration on membranes with 50–100 kDa cutoffs. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) showed that most host cell proteins were smaller than that. IgG was retained. Parallel concentration and buffer exchange could be performed going into a follow-on polishing step. These features made it an obvious candidate for initial capture, but it did not perform as hoped. Membrane fouling sabotaged its concentration–diafiltration potential, and prohibitive…
Large-Scale Purification of Factor-IX: Comparing Two Affinity Chromatography Resins
Human clotting factor-IX (F-IX) is a glycoprotein that is essential for normal hemostasis (1). A deficiency of F-IX in human plasma is caused by an absence or functional mutation of the F-IX gene that expresses inactive F-IX in plasma. That leads to hemophilia B (“Christmas disease,” named after its first identified patient), a genetic disorder in which the blood-clotting cascade is disturbed (2, 3). The structure and amino-acid sequence of F-IX are similar to those found in other vitamin-K–dependent glycoproteins.…
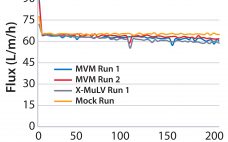
Advanced Viral Clearance Study Design: A Total Viral Challenge Approach to Virus Filtration
Biologics derived from mammalian organisms have been accepted for therapeutic use for almost a century (1). However, these pharmaceuticals have the potential for contamination with pathogenic adventitious agents such as viruses. With cell-line–derived recombinant proteins, the viral risks commonly include viruses in the Retroviridae and Parvoviridae families (2). As patient safety and manufacturing facility suitability became significant concerns in the 1980s and 1990s, several industry and regulatory bodies reached consensus on how to approach the unique challenges of viral safety…