Despite decades of advancement in characterization analytics, biotherapeutics still are largely defined by the manufacturing processes used to make them. This linking of process to clinical results (and thus to commercial success) has made the biopharmaceutical industry somewhat risk-averse when it comes to the adoption of new technologies. That desire to “derisk” biomanufacturing through better process understanding — as well as the need to adapt to uncertainties in patient population size through process flexibility — in turn drives the need…
Downstream Single-Use Technologies
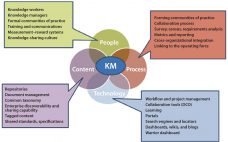
BioPhorum Operations Group Technology Roadmapping, Part 3: Enabling Technologies and Capabilities
Although great strides have been made over the past 20 years to increase the productivity and robustness of manufacturing processes for biopharmaceuticals, the cost and complexity of their development and manufacturing remain high, especially in comparison with those of small-molecule pharmaceuticals. Process improvements are required to increase patient access while maintaining the viability of an R&D-driven biopharmaceutical industry. Facility productivity, cost of goods (CoG), and capital investment all have significant margins for improvement. Such goals can be achieved not only…
Examining Single-Use Harvest Clarification Options: A Case Study Comparing Depth-Filter Turbidities and Recoveries
Steadily increasing demand for biopharmaceutical drugs has led the industry to examine its manufacturing scales while pressuring research and development groups to produce high-yielding clones and processes. Improved media, feed supplements, bioreactor designs, and control of process parameters have helped biomanufacturers achieve multifold increases in volumetric productivity from production bioreactors. However, cell culture processes are significantly affected by their bioreactor’s ability to support cells at higher densities and sustain cultures at lower viabilities. With the implementation of a number of…
Downstream Disposables: The Latest Single-Use Solutions for Downstream Processing
Downstream processing has been considered a “bottleneck” in the manufacture of protein biotherapeutics ever since cell culture engineers began dramatically improving production efficiencies around the turn of the century. And as single-use technologies have grown in importance and acceptance, offering more solutions every year, their biggest challenges too have been in the separation, purification, and processing that follows product expression in cell culture. Many of the technologies familiar to process engineers — e.g., centrifugation and chromatography — present technical and…
Single-Use Depth Filters: Application in Clarifying Industrial Cell Cultures
For current process development phases, many biomanufacturers’ attention is directed increasingly to the first unit operation in downstream processing, which is the removal of cells and cell debris from culture broth and clarification of supernatant containing a biopharmaceutical product. Given the high cell densities achievable with both mammalian and microbial cell culture processes, primary recovery can be a significant challenge. The current trend in cell culture is to increase product titers with enriched culture media, improved cell productivity, and increased…
Multicolumn Chromatography: Facilitating the Commercialization of Monoclonal Antibodies
Since 2001, global contract development and manufacturing organization (CDMO) CMC Biologics has completed more than 120 projects with at least 100 pharmaceutical partners. During that time, the company has taken a holistic approach to helping clients balance manufacturing risks and rewards. The team focuses on evaluating key technologies to deploy a constantly evolving set of capabilities in support of biopharmaceutical clients throughout their product lifecycles. Part of that commitment is continually evaluating what would best benefit customers and where key…
Membrane Adsorbers, Columns: Single-Use Alternatives to Resin Chromatography
Filtration membranes are used extensively throughout the biopharmaceutical industry for a range of applications, from coarse filtration to nanofiltration. Advantages of filter technologies include easy scaling, disposability, and (for many membrane filters) rapid and robust performance in a single-pass. The same advantages have been realized with membrane adsorbers. Chromatography resins are inherently disadvantaged by diffusion limits of the pores in chromatography media. Therefore, resin columns must be significantly oversized to match the performance of high productivity bioreactors. By comparison, membrane…
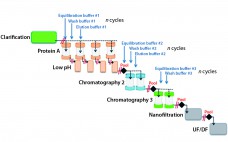
Accelerated, Seamless Antibody Purification: Process Intensification with Continuous Disposable Technology
Process intensification through continuous manufacturing has been practiced in the chemical, petrochemical, and food industries for years and has gained much interest among biopharmaceutical manufacturers (1). Key drivers encouraging biomanufacturers of therapeutic molecules to convert batch processes into continuous operation include flexibility, productivity, cost effectiveness, and product consistency. Continuous upstream processing has been demonstrated for the manufacture of a broad range of molecules, including complex/labile proteins such as enzymes (2) and monoclonal antibodies (3). Recent publications have reported successful application…
Diatomaceous Earth Filtration: Innovative Single-Use Concepts for Clarification of High-Density Mammalian Cell Cultures
In the past decade, biopharmaceutical manufacturers have demonstrated major improvements in monoclonal antibody (MAb) production, exhibiting product titers frequently in the range of 5–10 g/L using standard fed-batch mammalian cell cultures (1, 2). Increased product yields allow for smaller-scale production vessels. With 2,000-L single-use bioreactors already commercially available, single-use manufacturing of biomolecules becomes more and more an option. Other recent developments in the biopharmaceutical industry — e.g., drugs for smaller indications and more potent drugs allowing for lower dosages —…
Industry Experts Convene in New York to Discuss Latest Innovations: A BPI Special Report
As the biopharmaceutical industry continues to mature and grow, so too does the need to educate a broader audience of biopharmaceutical professionals interested in hearing, understanding, and applying the latest science and technology trends that support and in many cases are transforming today’s bioprocesses. To reach this extended and engaged audience, BioProcess International created the BPI Theater Series: a live, interactive program that provides bioprocessing content to traditional, noncore biopharmaceutical conference programs. It provides attendees with the opportunity to interact…