Biopharmaceutical manufacturing is evolving with the progression of Industry 4.0. Industry 4.0 refers to the ongoing “fourth industrial revolution,” which is transforming modern manufacturing and production practices through the use of “smart” technology. This is appealing especially to the biopharmaceutical industry, where production can be a long, meticulous, complex process, and optimizing manufacturing procedures is critical for success. As a leading supplier of single-use technology for the biopharmaceutical industry, PendoTECH recently has explored how its products can be integrated easily…
Downstream Single-Use Technologies
Validation of a Next-Generation Single-Use Turbidity System
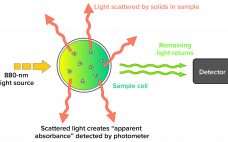
Turbidity describes the relative clarity of a liquid as the result of suspended solids. Instruments that measure turbidity typically use a beam of light to detect particles by measuring the difference between the amount of light emitted from the light source and the amount that is received by a detector. Such measurements are affected by the size, shape, and number of particles in a sample of liquid because those solids scatter the incoming light, which provides an apparent absorbance that…
The Downstream Perspective: Putting Product Knowledge to Work Using Technological Innovations
After over a quarter century in the industry — including downstream processing (DSP) and manufacturing directorships at Boehringer Ingelheim and leadership roles in technology development, quality, and manufacturing at Novasep — European consultant Margit Holzer is a recognized expert in downstream processing of biopharmaceutical products. Holding a doctorate in biotechnology from the University of Natural Resources and Applied Life Sciences in Austria, Holzer is familiar to BPI readers as both an author and conference participant (1, 2). And in May…
A Shift of Mindset: How Single-Use Systems Influence Bioprocess Engineering and Project Execution
In past decades, the focus in bioprocess engineering was on traditional stainless steel project management, which is highly dependent on established project schedules. Many milestones, tasks, and deliverables during basic and detailed design and execution were implemented in the same tried and tested way by engineering, suppliers, and biopharmaceutical companies. Making decisions was complicated by alignment with long lead items. With the introduction of single-use (SU) technologies, the transition in execution and optimization of project management only just has been…
Making Downstream Processing Continuous and Robust: A Virtual Roundtable
Current biomanufacturing is driven to pursue continuous processing for cost reduction and increased productivity, especially for monoclonal antibody (MAb) production and manufacturing. Although many technologies are now available and have been implemented in biodevelopment, implementation for large-scale production is still in its infancy. In a lively roundtable discussion at the BPI West conference in Santa Clara, CA (11 March 2019), participants touched on a number of important issues still to be resolved and technologies that are still in need of…
Scientific and Technological Advancements in Applications of Single-Use Technology: A Conference Report
Single-use technology (SUT) has been used increasingly both in clinical and commercial biomanufacturing (1). Proven major advantages include relatively low capital investment, elimination of batch-to-batch cross contamination and reuse cleaning validation efforts, flexibility in manufacturing, and shortened product lifecycles. However, some challenges and barriers to implementation remain: Consumables costs are increasing. Specific regulatory guidance is lacking, as is component interchangeability and standardization. And few if any leak-proof components/systems are available. International groups and associations focused on setting best practices and…
Intensification of Influenza Virus Purification: From Clarified Harvest to Formulated Product in a Single Shift
Influenza is a global respiratory disease with an estimated mortality of up to a half million people per year (1). The majority of traditional influenza vaccines are still produced in eggs. Downstream processing typically consists of clarification by centrifugation, concentration by ultrafiltration, and purification by ultracentrifugation (2). Recombinant vaccines are most often purified by chromatography. Chromatographic purification of viruses already has achieved major improvements in recovery and scalability (3), but it also is important because it enables virus purification to…
eBook: SUStainability — Concerning Single-Use Systems and the Environment
Disposable materials have been used in many aspects of biomanufacturing since muromonab was first launched in 1986. Single-use stirred-tank bioreactors first became commercially available from HyClone in 2004 (1). Despite their demonstrated value to bioprocessing, disposable materials remain the subject of wide-ranging differences of opinion. Discussions of any technology are healthy and important for identifying areas for improvement, but some hearsay and bold propositions made regarding single-use components and the environment are not always helpful. Sustainability is an important and…
A Two-Step Purification Process: Application of HIC Membrane Chromatography in a Disposable 2,000-L Clinical Facility
Given paradigm shifts in the biopharmaceutical industry over the past decade, product development timelines are squeezed as the number of molecules entering clinical development continues to increase. Manufacturing facilities, especially those supplying clinical trial materials, have had to adapt to this trend. One popular approach is to have fully disposable equipment that allows for quick product changeover and flexible manufacturing capacity to respond to variable clinical demand. Although many facilities-related technologies exist to support that concept (e.g., disposable probes and…
New Dimensions in Single-Use Filtration
Whether viral vectors are clarified or the bioburden after cell harvest needs to be reduced to recover antibodies, such applications in biopharmaceutical production require large filtration areas. Single-use technologies are indispensable in many such bioprocesses. Although some single-use filter assemblies have reached their limits, Sartorius Stedim Biotech has made developments to revolutionize these production steps. Scale-Up Limitations in Single-Use technology Conventional stainless steel process systems have been established for decades in the pharmaceutical industry. They are the basis of safe…