Cell-based potency testing provides quantitative data concerning a drug’s biological activity. Thus, it plays an essential role in biopharmaceutical quality control (QC), good manufacturing practice (GMP) product release, comparability determination, and stability testing for both drug products and drug substances. Potency is a critical quality attribute (CQA) often scrutinized by regulators and reviewers. Test methods are specific to a drug’s mechanism of action (MoA) and should be validated to internationally harmonized regulatory standards (1). The options preclude applying a simple…
Business
Embracing Innovation in Biomanufacturing
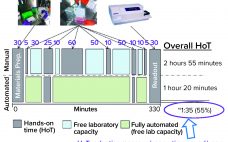
Innovations in bioproduction of therapeutics over the past 20 years have led to impressive improvements in product yield, process controls, and manufacturing safety. Industry 4.0 concepts have been embraced across the bioprocess industry and are leading to better bioprocess control through process automation, “big data” and data analysis, process simulations, the industrial internet of things (IIoT), cybersecurity, the cloud, blockchain/serialization, and additive manufacturing. Such advances help to ensure that a process results in the same outcome every time. As Sean…
The EU MDR Deadline Delay: What Does It Entail for Pharmaceutical Companies?
The life-sciences industry has been working hard to meet the deadline for compliance with the European Union’s Medical Device Regulation (EU MDR, 2017/745) (1). Doing so has been a challenging journey for many companies. Therefore, the full-year postponement of the final application date has been a welcome development, particularly in view of the new and extraordinary challenges stemming from the COVID-19 global health crisis. The extension has instigated other important changes, so it is critical that life-science businesses familiarize themselves…
Emerging Strategies for Drug Product Comparability and Process Validation: Part 1 — Analytical Tools and Drug Product Comparability
Process validation is a key part of the development and manufacture of all approved drug products, but its completion can be a daunting task. At a two-day CASSS CMC Strategy Forum held in July 2016 in Gaithersburg, MD, speakers and attendees addressed the many technical, practical, and regulatory facets of drug product process validation and comparability. In part 1 of this report, we summarize the key discussion points of the first day, which focused on analytics and comparability. Session One:…
COVID-19 As a Catalyst for Changing Orphan-Drug Regulations
A long-awaited (and for months withheld) evaluation of the European Union’s Orphan Drug Regulation (ODR) finally reached the interested public in August 2020 (1). Its publication during the public consultation of the European Commission’s “Pharmaceutical Strategy” was well planned because the latter discusses policies on access, availability, and affordability of new medicines. However, the ODR evaluation shows not only that the intentions behind the legislation have not been fulfilled, but also that its generous incentives (extension of market exclusivity and…
eBook: Vaccines — COVID-19 Invigorates a Stagnant Industry
The SARS-CoV-2 novel coronavirus has galvanized what was a stagnant and oligopoly-run vaccine industry. In this inaugural BioProcess Insider eBook, the first of four to be published in 2021, founding editor Dan Stanton explores economic and technical conditions that until now have hampered innovation in vaccine development and discouraged market entry for emerging biotechnology companies. Leveraging commentary from vaccine industry experts and analyzing the range of emerging vaccine modalities, Stanton surveys how industry responses to the COVID-19 pandemic are fostering…
Cold-Chain Validation: Emerging Vaccines for COVID-19 and Beyond Require More Extensive Evaluation
In the wake of fast-track approvals for Pfizer’s and Moderna’s respective SARS-CoV-2 vaccines, now begins the largest immunization campaign in world history. Its success will depend not only on the products’ safety and efficacy, but also on several mass-distribution programs requiring significant cold-chain infrastructure. The public has become acutely aware of the Pfizer vaccine’s demanding cryostorage specifications, generating considerable anxiety about how mass distribution will happen. Behind the scenes, however, cold-chain engineering companies such as Modality Solutions have worked alongside…
The Power of Industry Collaboration: Driving Harmonization of Regulatory Requirements
The biopharmaceutical industry continues to develop advanced manufacturing processes, systems, technologies, and facilities. Regulations play a significant role in assessing and approving marketing authorizations for drug products that are submitted for approval. The industry and regulators together should form a coordinated, streamlined process that delivers much-needed medicines around the world. Global Complications The complex global nature of biopharmaceuticals sometimes means that progress is not always as smooth as it could be. One main reason is that regulatory agencies often differ…
The Green Imperative: Part Two — Engineering for Sustainability in Single-Use Technologies
In BPI’s June 2020 issue, the first installment of this series introduces the study and implementation of single-use (SU) technology to provide a more sustainable manufacturing environment (1). We presented evidence showing that the economic and social benefits of SU systems currently outweigh the residual environmental risks. Not only is SU technology often a better environmental choice than traditional biomanufacturing options, it also is sometimes the only choice for rapid process design and facility start-up. In situations such as the…
Top Trends in Biomanufacturing
Facing an ongoing pandemic, growing pipelines, and a possible capacity crunch, the bioprocess industry is striving to balance its priorities. Those are some of the key issues to watch according to the 17th annual report and survey of biopharmaceutical manufacturing capacity and production from BioPlan Associates. It includes survey responses from 130 decision-makers (from 33 countries) at both bioprocessing organizations and contract manufacturing organizations (CMOs) and responses from 150 bioprocess industry suppliers (1). Top trends from this report are highlighted…