In an increasingly competitive life-science landscape that includes numerous mergers, acquisitions, and changing business models, the demand for collaboration is increasing at such a pace that it exceeds information technology (IT) capabilities. The need to manage and control this collaboration across the supply chain has become mandatory. That is particularly true for larger organizations with hundreds or thousands of partners that are finding new ways to connect, interact, and conduct business. Individual businesses are forming contractual affiliations that extend beyond…
Business
Biosimilars Awaken CROs
Biosimilars are revolutionizing the bioprocessing industry. Over the years, company CEOs have pushed different models, expanding and closing down huge amounts of internal manufacturing, research and development, and quality control facilities. Sometimes services were pushed to Asia, only to get brought back a few years afterward when a new CEO was appointed. Mergers and acquisitions further complicated those ideas. Consequently, many biopharmaceutical companies now have “gaps” in their drug development service provisions. Filling those gaps will fall on contract research…
Process Challenges of Antibody–Drug Conjugates
With two products now on the market, and a host of others in clinical trials, antibody-drug conjugates (ADCs) are slowly becoming a big business. Designed to deliver extremely active cytotoxic drugs that are otherwise undosable, they take advantage of the targeting ability of a specifically designed monoclonal antibody (MAb) to “shield” a highly potent API (HPAPI) as it travels through a patient’s bloodstream after administration. Once the antibody reaches its target on the cancer cell, it will release the payload,…
The Rise of Biopharmaceutical Outsourcing to Indian CDMOs
India is becoming an increasingly attractive destination for outsourcing biotechnology services by global biopharmaceutical companies. As “Big Pharma” continues on its path of finding ways to lower costs for development and manufacturing of biopharmaceuticals, Indian contract development and manufacturing organizations (CDMOs) are being viewed as capable and beneficial service providers that possess the necessary technical expertise and regulatory-compliant facilities. According to its 11th annual report on biopharmaceutical manufacturing capacity and production, BioPlan Associates ranked India fourth in the world as…
Qualification of Scale-Down Bioreactors: Validation of Process Changes in Commercial Production of Animal-Cell-Derived Products, Part 2 — Application
Here we apply our approach to validation of animal cell culture process changes using qualified, scale-down bioreactors. As described in Part 1 (including Table 1, Figures 1 and 2, and References 1–23), the goal is to facilitate implementation for the benefit of both the patients and industry. “Qualification of Scale-Down Bioreactors: Validation of Process Changes in Commercial Production of Animal-Cell–Derived Products, Part 1 — Concept” appears on pages 38–45 of BioProcess International’s May 2014 issue. Process changes often entail validation,…
Rapid Development and Scale-Up Through Strategic Partnership: Case Study of an Integrated Approach to Cell-Line and Process Development for Therapeutic Antibodies
Over the past decade, monoclonal antibodies have become mainstream therapeutics for treating a broad range of conditions from autoimmune disorders to cancer. Part of this evolution is increasing time and cost pressure on biopharmaceutical companies to bring new drugs to market 1, 2. Additionally, companies now routinely engineer and screen molecules for developability and manufacturability during discovery before selecting a final candidate molecule. The biosimilar development paradigm also demands significantly more bioanalytical analysis during initial cell-line and process development. Thus,…
Transformative Healthcare: The National Biomarker Development Alliance
A newly launched independent, nonprofit organization — the National Biomarker Development Alliance (NBDA) — will broadly engage leaders in industry, academia, patient groups, and government from across the United States. It was announced on 13 January 2013 at the National Press Club by the Research Collaboratory (RCASU) at Arizona State University (ASU). The mission of the NBDA is to address the complex and urgent challenge of creating standards needed for end-to-end, systems-based biomarker research and development (R&D). The alliance is…
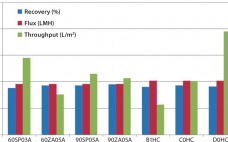
Strategies to Mitigate Technology Transfer and Clinical Manufacturing Risks: Downstream Purification Case Studies
Since the early 1980s, biotechnology products have been a fast-growing sector. They now occupy a significant portion of biologic drugs approved by regulatory authorities around the world every year. Among the approved biologic drug products, as well as those still in clinical testing, many are manufactured by contract manufacturing organizations (CMOs). Sponsor companies often transfer their developed process and process knowledge to CMOs for manufacturing of materials for toxicology and clinical studies. Drug Product Development Figure 1 illustrates the usual…
Risk Management in Financing of Capital Expansions: How One CMO Grows with Its Customers
BioVectra Inc. is an eastern Canadian contract manufacturing organization (CMO) with expertise in both synthetic chemistry and biomanufacturing techniques. In recent years, it has obtained specialized knowledge in production of highly potent small molecules from fermentation and functionalized methoxypoly(ethylene glycol) products (mPEGs). The focus of BioVectra’s contract manufacturing business is the transfer and scale-up of processes for manufacturing its clients’ products under current good manufacturing practices (CGMPs) as appropriate for the clinical stage of each product. In the current environment,…
Essentials in Quality By Design
Quality by design (QbD) is a systematic approach to drug development. It begins with predefined objectives and emphasizes product and process understanding and process control, all based on sound science, data-based decision making, and quality risk management (QRM). As introduced by the US Food and Drug Administration (FDA), QbD brings modern drug development methodologies to chemistry, manufacturing, and control (CMC) teams working on biologics, pharmaceuticals, and vaccines. The innovations associated with QbD are not so much the development concepts (which…