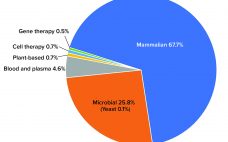
Cell and gene therapies (CGTs) offer potential cures to some of the most challenging illnesses of our time. The number of such therapies approved for market is set to surge in the next 10 years (1). Yet current manufacturing approaches are not fit for purpose. Biomanufacturing must adapt to prevent the industry from unintentionally sleepwalking into causing harm to patients. Some urgently needed changes could come with learning about the mindset of the medical-devices industry. Background and Current State After…
Business
Microbiome-Based Therapeutics: Negotiating Key Development Challenges
Microbiome-based therapeutics have evolved significantly in recent years, with several promising candidates advancing through the clinical pipeline. That progress is the result of growing evidence showing that targeting and manipulating the microbiome could improve human health and treat more than 25 conditions by restoring healthy bacterial populations (1). The human microbiome comprises a diverse community of microorganisms, helpful and harmful. It differs by person based on factors such as genetics, environmental influences, diet, and immune function. Microorganisms play a role…
The Next COVID Challenge: Building an Arsenal of Vaccines
For months, international partners have pressured the United States about intellectual property (IP) concerns and access to SARS-CoV-2 vaccines. As the country emerges from the worst of the pandemic, President Joe Biden now has an opportunity to shift his administration’s focus to realizing its longstanding promise of making the United States “the arsenal of vaccines for the world” (1). However, the country cannot accomplish that goal just by exporting mRNA vaccines and waiving IP protections. The current generation of vaccines…
eBook: Cell and Gene Therapies —
A 2021 Industry Update
The US Food and Drug Administration (FDA) reports that as of June 2021, 22 advanced therapy products have received regulatory approval in the United States. The first such product gained regulatory approval in 2010. Since then, hundreds of cell and gene therapies have advanced to clinical evaluation, but few products have reached commercial stages — and those that have done so have been hindered by manufacturing problems. In this eBook, writers from the BioProcess Insider and Project Farma analyze trends…
Mesenchymal Stromal Stem Cells: Next Steps and Considerations for CGMP Manufacturing
Massimo Dominici is scientific founder of Rigenerand srl, a joint venture between RanD (a biomedical company producing bioreactors for liver support and chemohyperthermic technology for cancer) and experts in cell and gene therapy at the University of Modena and Emilia Region in Italy. Rigenerand develops and manufactures medicinal products for cell-therapy applications (primarily for regenerative medicine and oncology) and three-dimensional (3D) bioreactors as an alternative to animal testing for preclinical investigations. The company also produces its own pipeline of cell…
Toward the Point of Care: Flexibility and Decentralization Are Key to Making Autologous Therapies More Readily Available
Part of the advanced therapy medicinal products (ATMPs) class of therapeutics, cell and gene therapies (CGTs) can be either autologous, using the patient’s own cells, or allogeneic, using master banked donor cells. Global biotechnology company Orgenesis focuses on autologous therapies, with processes and systems developed for closed and automated processing that have been validated for regulatory-compliant production at the point of care for patient treatment. This technology could help overcome the limitations of traditionally cost-prohibitive CGT manufacturing methods that do…
The Promise of Artificial Intelligence in Healthcare
The term artificial intelligence (AI) has become pervasive in conversations about the future of healthcare. AI has the potential to transform medicine through novel models of scientific discovery and healthcare delivery, ultimately leading to improved individual and public health. Yet misunderstanding and miscommunication abound. Thus, concepts related to AI need to be defined and explained to elevate our general level of understanding and our discourse around the topic. The Promise of AI in Healthcare AI has been studied by computer…
Risk Considerations for Aging Pharmaceutical Facility Cleanrooms
Pharmaceutical facility cleanrooms are designed to reduce and control particle contamination and to minimize the ingress and retention of microorganisms. Such risks typically are easy to control in well-designed, modern facilities. But risk mitigation is more difficult in older facilities. There is no exact definition of what constitutes an aging facility (or what are sometimes euphemistically called legacy facilities). For example, a facility established 100 years ago to manufacture a simple tablet can continue to operate perfectly well with careful…
Total Global Capacity Finally Shows Improved Productivity
Since 2018, global bioprocessing capacity has grown from 16.5 million liters (1) to 17.4 million liters. Although output has continued to expand at around 12% overall, that rate represents a significant slowing in capacity growth as the industry moves toward greater productivity and efficiency. Trends that we have tracked in the BioPlan Associates annual report of biopharmaceutical manufacturing capacity and production (2) for over 17 years correlate with that finding. Titers are increasing; single-use technologies have reduced the need for…
Facilities Roundup: What’s Behind the Expansions?
In the early 2000s, the trade press was abuzz about an imminent “capacity crunch” in mammalian cell culture. Dire predictions of shortages were based on biopharmaceutical successes to that point, on bursting development pipelines, and on the lengthy timelines and high costs of assembling tens of thousands of liters of stainless-steel bioreactors and supporting infrastructure. Those predictions failed to anticipate several positive developments that would render doom-and-gloom scenarios moot. Notably, yearly improvements in protein titers for MAb processes already were…