Johnson & Johnson’s pharmaceutical research arm recently launched a drug-development project that will intercept diseases before patients even realize they’re sick. In the company’s words: “The future of healthcare will increasingly depend on identifying and correctly interpreting the earliest signals of disease susceptibility, preventing or intercepting disease before it even begins.” (1). The first targets include Alzheimer’s, some forms of cancer, heart disease, rheumatoid arthritis, multiple sclerosis, and type 1 diabetes. The company’s Disease Interception Accelerator group will search for…
Business
Accounting for Portfolio Risk: Creating an Analytical Framework for Optimizing the Portfolio Mix
In the biopharmaceutical industry, portfolio risk is categorized as clinical/technical or commercial (Figure 1). Both types pose challenges to quantify. Portfolio managers tasked with optimizing the mix of products in a company’s pipeline often struggle to create apples-to-apples frameworks that can consistently compare risk across asset categories. The framework described herein can help you account for both technical and commercial risk using simple analytical tools. The foundational analytical element of portfolio management is traditionally the asset forecast: a composite best…
From Silos to Synergy: Optimize Your Managed-Markets Division with Cross-Team Collaboration
Like many other industries, life sciences faces rapid, technology-driven changes and a shifting business environment. Many patients are covered by Medicare, Medicaid, or national healthcare organizations outside the United States. That necessitates pricing and reimbursement negotiations that are complicated by rules and regulations. Patients whose health plans belong under a managed-care organization (MCO) or pharmacy-benefit manager (PBM) have access to prescriptions primarily through staff-model pharmacies, mail-order pharmacies, and network pharmacies. Drug manufacturers offer incentives to such organizations in the form…
Standardization of Disposables Design: The Path Forward for a Potential Game Changer
Recent articles have described how the debate on standardization is slowing down adoption of single-use technology (1). The Standardized Disposables Design (SDD) initiative is working to design simple standard single-use solutions for real-life examples (e.g., buffer bags). In reality, a buffer is a buffer whether it is made in Europe, Asia, or America, so in essence different solutions are not necessary for different end users. A buffer bag is not difficult to design, and it does not vary greatly in…
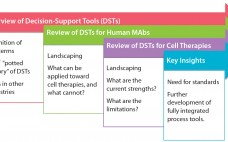
Decision-Support Tools for Monoclonal Antibody and Cell Therapy Bioprocessing: Current Landscape and Development Opportunities
Industrial-scale manufacturers in a number of fields — from automobiles to biotherapeutics — have long relied on powerful computational and mathematical tools to aid in the scale-up, optimization, quality control, and monitoring of product development (1–5). Typical process pathways are highly multifactorial, with numerous branch points, feedback steps, instrumental attributes, and target parameters. Moreover, margins for error are minimal for most industrial processes, requiring high standards of precision from industrial and operational pathways (6). For those reasons, the complexity of…
Elucidation: Centers for the Innovation in Advanced Development and Manufacturing
The 2009 influenza pandemic was the main driver for the Centers for the Innovation in Advanced Development and Manufacturing (CIADM). As a new federal government authority, the Biomedical Advanced Research and Development Authority (BARDA), directed by Dr. Robin Robinson, assembled a team of scientists and technicians with a vast array of knowledge and experience in chemical, biological, radiation, and nuclear fields. BARDA’s directive was to enhance preparedness for catastrophic challenges such as an influenza pandemic or other serious threats to…
A Risk-Based Approach to Supplier and Raw Materials Management
Ensuring a continuous supply of safe medicines is a key objective for the pharmaceutical industry and health authorities alike. A critical component to that end is maintaining a reliable supply of qualified raw materials (RMs) used in drug production. However, changes in suppliers, their processes, their providers, and consequently the materials they supply can occur (for a number of reasons) at any time during the life cycle of drug production. A product-supply organization therefore must be prepared to address such…
Next-Generation Bioprocessing for Meeting Healthcare Challenges: The Role Single-Use Handling Systems Can Play
The rapid spread of contagious and lethal diseases worldwide has driven bioprocess suppliers to develop technologies for use in producing disease treatments and vaccines. Bioprocessors need to develop new biologics as well as rapid and reliable methods for bringing those treatments to commercialization. Implementing modular process solutions and single‑use handling systems in closed‑manufacturing processing is one approach to addressing those needs. Developing and discovering solutions for meeting global healthcare conditions is an evolving part of bioindustry. As points of reference,…
Special Report on Assays, Test Methods, and Comparability: The CMC Strategy Forum Series, Part 4, Introduction
The CMC Strategy Forums focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of the forum meetings are published in BioProcess International and on the CASSS website (www.casss.org). This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated environment will continue…
Special Report on Assays, Test Methods, and Comparability The CMC Strategy Forum Series, Part 4, Biosimilar Products: Scientific Principles, Challenges, and Opportunities
The Chemistry, Manufacturing, and Controls (CMC) Strategy Forum held on 22 January 2012 in San Francisco, CA, focused on selected scientific and regulatory aspects in the development of biosimilar products. Such products are an increasingly important area of interest for both the biopharmaceutical industry and its regulatory agencies. Biosimilars are highly complex, so scientists have been unable to demonstrate identity to a level typically possible for small molecules. Consequently, specific scientific and regulatory approaches are required to ensure the high…