As the global pharmaceutical industry implements serialization (track and trace from manufacturing to dispensing) to meet governmental regulatory requirements, other opportunities arise for drug companies. The main driver here is to improve the integrity of the overall drug supply chain, but other meaningful business benefits can come from serialization. Generally accomplished through automated, electronic means, it involves such practices as recording, authenticating, maintaining, and sharing accurate records of products. Outside-the-Box Benefits In addition to tightening up the supply chain and…
Business
eBook: Of Microbrews and Medicines — Understanding Their Similarities and Differences in Bioprocessing Can Help Improve Yields and Quality While Reducing Cost
Meeting a biopharmaceutical scientist or engineer who proclaims a love for brewing is not surprising. Perhaps itâs because of the challenge of mixing raw ingredients together and waiting patiently for the final product, maybe itâs the hands-on nature of the equipment or the data analytics entertainment, or it just might be the simple joy of creating something. Whatever attracts a scientist or engineer to making medicines and/or craft brews, a surprising number of principles hold true for both bioprocesses despite…
Postapproval CMC Changes: Increasingly a Fact of Biopharmaceutical Life
The manufacture of vaccines and therapeutic proteins has suffered from a reputation of being part art and part science, with heavy doses of regulatory uncertainty thrown in. Postapproval changes (PACs) to chemistry, manufacturing, and controls (CMC) were initiated reluctantly and carefully in the era of âthe process is the product.â Today, CMC PACs are a normal part of the biopharmaceutical industry business. Emma Ramnarine (head of global biologics quality control at Hoffmann-La Roche in South San Francisco, CA) notes that…
eBook: Challenges Facing Biosimilar Entries into US Markets
Since the 2009 enactment of the Biologics Price Competition and Innovation Act (BPCIA) (1), the US Food and Drug Administration (FDA) has licensed six biosimilar products under PHS 351(k) and approved one product under FD&C 505 (b)(2). It also provided complete response letters (CRLs) to four biologics license application (BLA) filings (Table 1) (2). By comparison, the European Medicines Agency (EMA) has approved 31 biosimilar products (3) and refused or withdrawn about five. There is no doubt that US market…
Serialization: Background, Justification, Requirements, Timelines, and Readiness Across the Supply Chain
Drug manufacturers are facing unprecedented serialization challenges. Serialization requires weighty consideration and focused strategy for successful commercialization, even for those companies that have yet to bring a product to market. The World Health Organization estimates that 10% of medicines worldwide and up to 50% of drugs consumed in developing nations are counterfeit. In response to increasing drug integrity concerns, more than 40 countries have introduced laws mandating serialization and tracing of pharmaceutical products as they pass through the supply chain.…
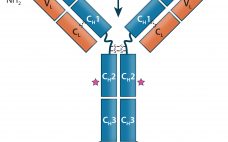
Therapeutic IgG-Like Bispecific Antibodies: Modular Versatility and Manufacturing Challenges, Part 1
Antibody-based immunotherapy has advanced significantly since 1986, when the US Food and Drug Administration (FDA) approved the first mouse monoclonal antibody (MAb) for clinical use: Orthoclone OKT-3 (muromonab-CD3). In the intervening years, researchers have applied the tools of genetic engineering to clone immunoglobulin G (IgG) genes into a number of expression vectors. In the 1990s, the bioprocess industry was able to produce fully human antibodies in cultured cells. As of June 2017, the FDA and the European Medicines Agency (EMA)…
Data Analysis and Visualization to Improve Biopharmaceutical Operations Part 1: What Are You Trying To Measure?
This begins a five-article series of âhow-toâ guides for tackling the most common obstacles in assessing, measuring, analyzing, and improving the performance of global biopharmaceutical manufacturing operations. Each installment covers a component of proper collection, analysis, and use of data for the best possible performance outcomes. When taken as a whole, the series should provide imperative best practices for handling business-performance data. First, consider what you want to know about your bioprocesses. How can you more appropriately measure those data…
Better Solutions Needed As Cancer Drug Costs Escalate
The out-of-pocket price of many life-saving cancer medications continues to rise while insurance companies continue to raise deductibles and copays. Patients are paying more for their prescriptions than ever before, and they need solutions that offer cost-effective treatment. The Oncological Problem Cancer is the second leading cause of death in the United States. More often than ever before, patients obtaining potentially life-saving cancer drugs face a severe financial burden (1). Newer cancer medications can cost patients over US$100,000 each year,…
Building Competency in Basic Science: The Secret Weapon of Tomorrowâs Bioprocessing Technician
Todayâs bioprocessing technicians are highly skilled professionals who can operate large automated equipment, juggle numerous support activities, and document manufacturing deviations. In coming years, their jobs will become even more rigorous as companies push more decision-making to the production floor to save time and resources. With the trend toward smaller batches made in bench-scale and/or single-use equipment, this strategy becomes easier to implement. One way to foster improved decision-making on the production floor is to hire or promote employees who…
Addressing Knowledge Gaps and Skills Development: Modular Training Keeps the Bioindustry at the Leading Edge
One major challenge facing the global bioindustry today is finding talented individuals to work in the type of highly skilled interdisciplinary environments necessary for effective bioprocess development. Ideally, such individuals require a combination of technical knowledge and expertise spanning biological sciences, physical sciences, mathematics, and engineering. Numerous industry surveys have repeatedly stressed the lack of suitably trained individuals equipped with necessary skills to work at the biologyâengineering interface to meet the growing and changing demands of industry. The challenge is…