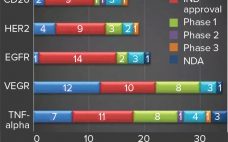
One of the first steps toward understanding how to patent an invention, invalidate someone else’s, or enter a new area of research is conducting a patent search. Knowing whether your invention is similar to those that already have been reported is critical before applying for a patent or beginning research. If your invention has been patented already or has been described or published anywhere in the world, then it is considered “prior art,” and your patent will be denied. Numerous…
Business
Emerging Treatments for Spinal Cord Damage
Injuries to the spinal cord can cause permanent paralysis and even lead to death, with little or no hope for patients to regain lost function after such trauma has occurred. News of my spinal cord research first came to prominence in the 1980s with a front-page story that chronicles a presentation at the annual Society for Neuroscience meeting (1). That reported the first time that crushed peripheral nerves had been regenerated back into a patient’s spinal cord. Regeneration of spinal…
Biopharmaceutical Growth in China: Bioprocessing Challenges Are Creating CMO Opportunities
In the past decade, the world has seen rapid growth of more than 15% in China’s biopharmaceutical market (1). According to the second edition of BioPlan’s Advances in Biopharmaceutical Technology in China, the most populous country in the world — with the largest patient groups — has a growing economy with its GDP second only to that of the United States. Rapid urbanization and greater access to national healthcare insurance puts China in second place after the United States for…
Toward Standardized Sample Collections: UK Government Seeks to Expand Cell and Gene Therapy Use
The SAMPLE program — a Standard Approach to ATMP Tissue collection — is intended to help build capacity for the UK National Health System (NHS) to expand the use of next-generation cell and gene therapies for both cancer and noncancer illnesses. Now it has been given the green light by Innovate UK (IUK), the United Kingdom’s technology strategy agency. An award through the Industrial Strategy Challenge Fund is supporting the program for standardizing how cell and gene samples are collected…
Good Manufacturing Practice in China: Equipment Strategy and Quality Management to Compete with the West
Although the concept of good manufacturing practice (GMP) was created about half a century ago in the United States, the history of GMP biomanufacturing is relatively short in China, with the first version of GMP rules issued by the Ministry of Health in 1998. In 2010, the ministry issued its fourth version of China’s GMP rules, which came into effect in March of 2011 (1). According to a recent report from BioPlan Associates, the 2010 version raised the standards to…
eBook: Vaccines – Industry Collaborations to Increase Uptake
Vaccines save millions of lives every year, and the continuing increases in the number of administered doses and worldwide distribution of vaccines for long-standing diseases such as polio and malaria have contributed to the improvement in public health. Vaccine developers and manufacturers are partnering with private and government agencies to raise global vaccine uptake by addressing remaining challenges with production capacity, distribution, safety, and accessibility. And the implementation of new technologies such as virus-like particles and cell/DNA-based vaccines are helping…
Why Conducting Marketing Due Diligence Early in Product Development Is Important
To be successful, a company needs two main ingredients: good science and good business/ marketing. Without good science, a product won’t work, and without a good marketing strategy, a product won’t sell. Two important questions should be addressed: When should marketing groups be involved in product development, and how important is that? The answer to the first is as early as basic research. Why? After a product is launched, a biomanufacturer doesn’t want to discover that its product applies only…
Breaking Through the Noise: An Approach to Differentiating Your Business
When multiple businesses sell the same type of item, why do customers buy from one supplier rather than another? How are vendors able to break through the “white noise” of an industry to stand apart and get noticed? The current bioprocessing and cell therapy vendor markets, about 50,000 vendors are selling to biomanufacturers, universities, and research institutions (www.bioz.com). How do those suppliers get noticed? Market-leading suppliers have established brands that allow them to cross-sell, up-sell, and engage in deep selling.…
China Looks Inward and Outward: Investments in US and EU Biopharmaceutical Companies Are on the Rise
The growth momentum of China’s biopharmaceutical industry continues, with the China Industry Research Institute projecting that the country’s biological therapeutics market could reach 300 billion Chinese yuan (~US$50 billion) in 2019. According to the second edition of our report on advances in Chinese biopharmaceutical technology, this robust growth is boosted by continuing investment into the sector (1). A clear strategic indicator of the country’s intentions in biotherapeutics and biologics has been government investment in bioindustrial hubs, which by next year…
Biologic Labels and Induced Patent Infringement: A Perspective on Evolving US Law
The mechanism for proving patent infringement is changing for developers of both branded and follow-on biologics (either biosimilar or interchangeable). Here we examine how drug labeling can establish infringement, thus affecting follow-on manufacturers accused of inducing others to infringe patents on methods of treating medical conditions. Because precedent is paramount in the US legal system, judges look to small-molecule case history to help them understand alleged infringement by follow-on biologics. The classic approach to induced infringement of generic small-molecule drugs…