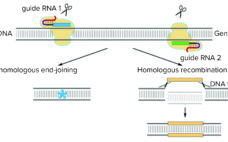
Since January 2016 (with a brief interlude as described below), the Patent Trial and Appeal Board has been attempting to adjudicate the proper inventorship of CRISPR technology in (to date) six separate patent-interference proceedings. (Scientific “priority has been decided, for now, by the awarding of the Nobel Prize in Chemistry to Jennifer Doudna and Emmanuelle Charpentier in 2020; see the “Priority Claims” box). CRISPR-based gene editing was hailed as the “Breakthrough of the Year” in 2015 (1), and the scientific…
Intellectual Property
Patent Thickets Constrain US Biosimilars Market
Biosimilars represent a significant cost-savings opportunity in the United States because they can introduce competition for some of the most expensive and widely used prescription drugs on the market: originator biologics. Several market and regulatory barriers have slowed biosimilar market entry and uptake in the United States (1). Some hurdles are unintentional or transitory; others are deliberately crafted by manufacturers of reference biologics to thwart competition. Among the most significant strategic barriers to biosimilars’ entry into the US market are…
Collaboration Agreements: Critical Issues and Common Pitfalls
The number of collaboration arrangements for emerging technologies is increasing significantly, especially among companies developing COVID-19 diagnostics, vaccines, and therapeutics. Consequently, a massive amount of capital was invested in the biopharmaceutical industry in 2020. These deals range in value from the low millions to several billion dollars, representing significant risks and value for investors. Some recipients of that capital are seeking partners with whom to collaborate, sharing both the costs and risks associated with their activities and bringing innovative technologies…
Intellectual Property and COVID-19
The COVID-19 pandemic is the greatest global health threat caused by a virus since the influenza pandemic of 1918 (and, before that, innumerable smallpox outbreaks throughout history). So far, the number of infections and deaths has not reached levels seen during the “Spanish flu” pandemic. However, travel, global trade, and modern factors such as misinformation on social media have increased infection rates and risks of infection. As of 15 July 2021, the World Health Organization (WHO) had reported 188,655,968 confirmed…
Trade-Secret Vulnerabilities: Recent Hacking Schemes Highlight the Need to Protect Proprietary Pharmaceutical Information
On 21 July 2020, the US Attorney’s Office in Spokane, WA, unveiled a sweeping indictment accusing a pair of Chinese hackers of conspiring to steal trade secrets from a number of American pharmaceutical companies and research institutions, including biotechnology companies working on potential COVID-19 vaccines (1). Although the hackers apparently did not succeed in their attempt to obtain vaccine data, their years-long conspiracy involved other attacks on the pharmaceutical industry — and netted them troves of sensitive commercial information. Among…
Biosimilars Pipeline and Market Trends
Most biopharmaceutical industry experts now consider biosimilars to be mainstream products, indicating that the field has progressed immensely over the past 10 years. Nevertheless, when comparing approvals and commercial offerings across the globe between 2013 and 2020, it becomes clear that some regions welcome these therapies more than others do. Western European biosimilars markets continue to be kind to these drugs’ production, distribution, and coverage; and companies headquartered in Asia and the Pacific Rim increasingly are getting involved in biosimilars…
Cell and Gene Therapies Get a Reality Check: A Conversation with Anthony Davies of Dark Horse Consulting Group
As founder of cell and gene therapy (CGT) specialist firm Dark Horse Consulting Group in California, Anthony Davies speaks from a quarter century of experience including former positions at Onyx Pharmaceuticals, Syrrx, ZymeQuest, Serologicals, Geron Corporation, Capricor, and 4D Molecular Therapeutics — and he currently serves on the board of directors for TrakCel and the scientific advisory boards for Akron Biotech and BioLife Solutions. In his plenary address at the Phacilitate 2020 Leaders World conference (part of Advanced Therapies Week…
eBook: Joining Forces — Industry Collaborations Toward BioProcess Success
Companies in the biopharmaceutical industry increasingly are working together to solve the many challenges of product/process development and biomanufacturing. Suppliers seek end-user help in refining technologies; academics and small innovators attract the financing and business acumen of large companies; equal partners share in technological problem-solving; and sponsors engage the development expertise of contract research and manufacturing organizations. Other examples of biopharma industry collaborations abound, too. Citing critical examples from the September 2019 BioProcess International East Conference in Boston, MA, this…
Demystifying the Patent Search Process — Revisited
One of the first steps toward understanding how to patent an invention, invalidate someone else’s, or enter a new area of research is conducting a patent search. Knowing whether your invention is similar to those that already have been reported is critical before applying for a patent or beginning research. If your invention has been patented already or has been described or published anywhere in the world, then it is considered “prior art,” and your patent will be denied. Numerous…
Biologic Labels and Induced Patent Infringement: A Perspective on Evolving US Law
The mechanism for proving patent infringement is changing for developers of both branded and follow-on biologics (either biosimilar or interchangeable). Here we examine how drug labeling can establish infringement, thus affecting follow-on manufacturers accused of inducing others to infringe patents on methods of treating medical conditions. Because precedent is paramount in the US legal system, judges look to small-molecule case history to help them understand alleged infringement by follow-on biologics. The classic approach to induced infringement of generic small-molecule drugs…