The quality by design (QbD) modernized approach to pharmaceutical development is intended to provide regulatory flexibility, increased development and manufacturing efficiency, and greater room to innovate as well as improve manufacturing processes within defined ranges without obtaining regulatory approval first. QbD is a systematic developmental approach that starts with a clear goal in mind and emphasizes understanding of how variability in both process and materials affects a final product (1). Historically, product quality has been assured either with end-product testing…
CMC Forums
Methods on the Move: Addressing Method Transfer Challenges for the Biopharmaceutical Industry
Analytical method transfers are essential components of the current global biotechnology environment. Analytical method transfer can be defined as “a documented process that qualifies a laboratory (the receiving laboratory) to use a validated analytical test procedure that originated in another laboratory (sending laboratory), thus ensuring that the receiving laboratory has the procedural knowledge and ability to perform the transferred analytical procedure as intended” (1). The goal is to ensure that a method continues to perform in the validated state regardless…
eBook: Production Cell-Line Development and Control of Product Consistency During Cultivation — Myths, Risks, and Best Practices
Health authorities are requesting substantial details from sponsors regarding practices used to generate production cell lines for recombinant DNA–(rDNA) derived biopharmaceuticals. Authorities also are asking for information about the clonality of master cell banks (MCBs) and control strategies to minimize genetic heterogeneity. Such requests are prompted by recent reports indicating “nonclonality” for certain production cell lines. To address these and related issues, the CASSS CMC Strategy Forum on “Production Cell Line Development and Control of Product Consistency During Cell Cultivation:…
Change Happens: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management (CMC Forum)
In the current global regulatory environment, management and implementation of postapproval CMC changes often can be unpredictable and inefficient. Timelines for change approval can vary from months to years, depending on regional regulatory procedures. Therefore, the challenge in postapproval lifecycle management is to maintain a constant supply of high-quality product while supporting innovation and continual improvement. This was the premise of the CASSS Chemistry, Manufacturing, and Controls (CMC) Strategy Forum held in Gaithersburg, MD, on 20–21 July 2016. The forum…
Strategies for Successful Sample Transfer
Nadine Ritter is president and senior analytical advisor of Global Biotech Experts, LLC and a long-time member of BioProcess International’s editorial advisory board. At a recent CASSS North American CMC Strategy Forum called “Methods on the Move: Addressing Method Transfer Challenges,” she discussed the biopharmaceutical industry’s logistical challenges of analytical test samples for drug substances and products. At the conference, BPI’s editor in chief Anne Montgomery met with her to discuss some key points of this topic. Logistics Challenges Montgomery:…
CMC Strategy Forum on Combination Products for Biopharmaceuticals: Emerging Trends in Development, GMPs, and Regulatory Expectations
On 26 January 2015, CASSS hosted a program in its ongoing series of semiannual Chemistry, Manufacturing, and Controls (CMC) Strategy Forums at the Mayflower Hotel in Washington, DC. Since this series’s inception in 2002, each installment has focused on one of a wide array of topics spanning the fields of biopharmaceutical product development, manufacturing, analysis, quality, and regulation. For this forum, the program committee chose to devote a full program to a topic that was previously the focus of an…
CMC Forum: Evolution of Biopharmaceutical Control Strategy Through Continued Process Verification
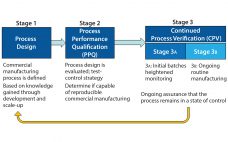
As defined in the ICH Q10 guideline, a control strategy is “a planned set of controls, derived from current product and process understanding, that assures process performance and product quality” (1). Every biopharmaceutical manufacturing process has an associated control strategy. FDA’s 2011 guidance for process validation (2) describes process validation activities in three stages (Figure 1). A primary goal of stage 1 is to establish a strategy for process control that ensures a commercial process consistently produces acceptable quality products.…
Special Report on Process- and Product-Related Impurities (A CMC Strategy Forum Special Focus Series): Extractables, Leachables, Particles, and Aggregates
The CMC Strategy Forums focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of forum meetings are published in BioProcess International and on the CASSS website (www.casss.org). This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated environment will continue to…
Science, Risks, and Regulations: Current Perspectives on Host Cell Protein Analysis and Control
State-of-the-art analytics guide process development by providing companies with thorough understanding, effective removal, suitable control, and comparability assessment after process changes of host cell proteins (HCPs) in recombinant biotechnology products. An array of analytical techniques and approaches can be used to establish control strategies for host cell proteins. Techniques used for HCP characterization and comparability include two-dimensional (2D) gel electrophoresis with a range of stains, 2D immunoblotting, 2D high-performance liquid chromatography (HPLC), 2D difference gel electrophoresis (DIGE), and increasingly mass…
CMC Strategy Forum Special Focus Series: Part 2 Product-Related Impurities, An Overview
Introduction by Cheryl Scott The CMC Strategy Forums focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of forum meetings are published in BioProcess International and on the CASSS website. This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated environment…