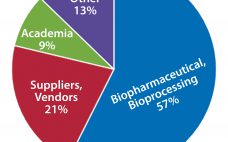
Training is an investment. And it’s one that the authors, contributors, and other individuals behind this supplement understand well. However, the biopharmaceutical industry is much larger than these few individuals. It’s just as important to understand the motivators, drivers, and training-related challenges faced by the industry at large. So BPI and BTEC jointly administered a short survey to gather some basic information from the magazine’s readership about the value and role that training plays in their organizations. According to survey…
Careers
Hiring and Staffing in Biopharmaceutical Manufacturing: Five-Year Trends Indicate Difficulty in Filling Positions
The biopharmaceutical manufacturing industry continues to expand rapidly, with growth in revenue for both suppliers and drug innovators averaging about 15% per year. Understandably, much of the industry’s recent focus has been on the pace of technological improvements that have boosted productivity and performance. But another issue is becoming just as critical: The flow of skilled staff able to keep up with the industry’s growth isn’t keeping pace. Results from our 2015 annual industry study suggest that the hiring market…
From Silos to Synergy: Optimize Your Managed-Markets Division with Cross-Team Collaboration
Like many other industries, life sciences faces rapid, technology-driven changes and a shifting business environment. Many patients are covered by Medicare, Medicaid, or national healthcare organizations outside the United States. That necessitates pricing and reimbursement negotiations that are complicated by rules and regulations. Patients whose health plans belong under a managed-care organization (MCO) or pharmacy-benefit manager (PBM) have access to prescriptions primarily through staff-model pharmacies, mail-order pharmacies, and network pharmacies. Drug manufacturers offer incentives to such organizations in the form…
Comprehensive Hands-On Training for Biopharmaceutical Manufacturing: BTEC’s Program to Deliver Training to FDA Investigators
Training and continuing education play a vital role in carrying out the US Food and Drug Administration’s mission to protect and promote the public health — not only for consumers, health professionals, and industry, but also for the agency’s own personnel. Since 2008, the Golden LEAF Biomanufacturing Training and Education Center (BTEC) at North Carolina State University has filled a niche in the agency’s internal training program and provided a series of courses to more than 100 FDA investigators. The…
Elucidation: The Medical Products Industry Must Resist the Pharmaphobes
I often ask people: “Do you think medical practice is better, worse, or the same compared with what it was when I graduated from medical school nearly 50 years ago?” The most common answer is “I guess it’s somewhat better,” although some say “Worse!” I explain that the unequivocal right answer is “hugely better!” For example, our average lifespan is now a decade longer, and we suffer far less from incapacitating diseases such as arthritis. Then I ask, “Why are…
Information Instead of Data: User-Friendly HMI Concept Increases Process Control Efficiency
Today, most plant operators are much more than classic process operators. In addition to operational process control, their range of tasks includes product quality assurance, optimization of resources, and maintenance of high‑throughput rates. Qualified information is necessary to reliably perform those tasks. The human–machine interface (HMI) of a process control system provides visualization. Siemens regards new HMI concepts such as advanced process graphics (APG) as the key to task‑specific or situation‑specific decision‑making (Photo 1). Graphics Monitor: the Process Window Before…
When Is a Virtual Business Model Suitable for Biopharmaceutical Companies?
Virtual companies are based on the model that all activities are outsourced. Such companies have no (or few) employees, occupy no laboratory space, and use contract service organizations for all activities. Over the past decade, several virtual biopharmaceutical companies have formed (1–4). They are primarily start-up ventures that use contract research organizations (CROs) for R&D and contract manufacturing organizations (CMOs) for product manufacturing. By contrast, a fully integrated biopharmaceutical company is based on the model that all activities are internal to…
A Case for Biopharmaceutical Operations in Low-Cost Countries to Maximize Return on Investment
A company’s success is associated with its growth. An expanding company hires more employees (full-time equivalents, FTEs) to support its commercial products, to develop new products, and to provide administrative services. As it grows, an organization can become more complex and its operations less efficient. Analysis of 2013 financial data showed that revenue of US biopharmaceutical companies increases with a growing number of FTEs at a slower rate than does cost (1). That study assumed that all US biopharmaceutical companies…
Entrepreneurship, Lessons Learned, and Bioindustry Perspectives: An Interview with Roger-Marc Nicoud
Roger-Marc Nicoud received his PhD from the University of Lorraine in process simulation for the nuclear industry. He joined Separex in 1987, first as a technical director and then as a managing director until 1995. Between 1993 and 1995, he also worked as professor and headed a research laboratory involved in thermodynamics at the University of Lorraine. Nicoud founded Novasep in 1995 with the objective of developing comprehensive solutions for producing biologic and synthetic molecules. Novasep became a leading life-sciences…
Process Effectiveness Analysis Toward Enhanced Operational Efficiency, Faster Product Development, and Lower Operating Costs
Complex drug development and biomanufacturing processes involve back-and-forth shuttling of activities among multiple functions. Close communication, collaboration, and coordination among stakeholder departments and functions are needed to successfully execute these processes. Whereas collaboration between multiple functions leverages each function’s expertise, the resultant structure also poses several challenges, as listed in Table 1 (1). These challenges are further exacerbated as an organization grows in size and geography (2, 3). In the absence of clarity and appropriate assignment of roles and responsibilities,…