Here we review strategies for gaining Food and Drug Administration (FDA) approval of allogeneic, pluripotent cell therapies. The crux of the discussion is that when developing a cell therapeutic, it is critical to look as much as a decade ahead to when FDA approval will be sought to commercialize the product through a biologics license application (BLA). While this discussion focuses on FDA approval of cell therapies, it is important to acknowledge the vast number of cell therapy clinical efforts…
BPI White Papers
Your Guide to Efficiently Develop Antibody-Based Therapeutics
This eBook presents various techniques used to measure the stability of antibody-based biotherapeutics. The authors address the ins-and-outs of monoclonal antibody (MAb) formulation, cover Investigational New Drug (IND) and New Drug Application (NDA) filing timelines, and explain how storage conditions affect MAb stability. Monoclonal antibodies have been used in therapeutics for more than 30 years. Efforts to further develop and optimize better MAb-based biotherapeutics are increasing as they have become increasingly popular for the treatment and prevention of many diseases…
Scalability of Lentiviral Production with the CTS LV-MAX Lentiviral Production System in Bioreactors
The Gibco™ CTS™ LV-MAX™ Lentiviral Production System provides a scalable and high-yield lentiviral vector (LV) production platform. It is based on a high-density suspension culture of HEK293F‚Äďderived Viral Production Cells that have been optimized for viral production in chemically defined LV-MAX Production Medium. Scalable LV production of greater than 1 x 10‚Āł TU/mL LV (unconcentrated) can be achieved using our proprietary lipid nanoparticle transfection reagent in combination with an LV-specific enhancer and production supplement. All components work synergistically to help…
Technology Transfers РA Beginner’s Guide
Pharmaceutical developers are increasingly relying on external manufacturing partners for expertise to develop and commercialize their products. In fact, contract development and manufacturing organizations (CDMOs) now process and manufacture approximately 28 percent of the world‚Äôs prescription and non-prescription drugs. Innovation is coming from all corners of pharma including independent labs, scientific consortia, academia and government programs. These emerging sources of drug innovation often have excellent science but few resources and a lack of experience in commercializing formulations and drug product…
Future Proof Your HCP Strategy
Future proof your HCP strategy. Four questions to help you decide if your HCP analysis data is ready for next phase approval. Host cell proteins (HCPs) are a primary source of impurities in biologics development. Scientists must detect and remove HCPs to ensure patient safety and meet regulatory guidelines. If previously undetected HCPs are found in later phases of clinical trials, scientists will need to redesign the drug substance purification strategy ‚Äď adding financial and time costs that can create…
Automated PBMC Isolation and T Cell Wash and Concentration by the CTS Rotea System
Successful processing and manufacturing in cell and gene therapy workflows are essential to the efficacy of the product. Autologous cell and gene therapy workflows involve isolating cells from an individual, engineering the cells, expanding and concentrating them, and infusing them back into the patient. Certain steps in these workflows could benefit from optimized automation to decrease hands-on time and the cost of the cell manufacturing process. In this application note, we present the Gibco™ Cell Therapy Systems™ (CTS™) Rotea™ Counterflow…
Do Not Let HCPs Delay Your Biologics Route to Market
The stakes are high in biologics development, especially in host cell protein (HCP) analysis. Regulatory bodies have rules and suggested strategies for HCP analysis and reporting, and it is likely that removing HCPs is a key concern in your process development. Ultimately, the accuracy of monitoring and success of purifying your biologic of these HCPs can make or break your product. Get it wrong and it could delay your development cycle. In this blog, we outline strategies to optimize your…
Ensuring Everything is A-OK at AKBA
Accurately and reliably producing pharmaceuticals and biotherapeutics is one of the most important ‚Äď and challenging ‚Äď tasks in the world of manufacturing. Any misstep in the production process will lead to the creation of a substandard end product that can do substantial harm to the user. That‚Äôs why the processes used in these production chains ‚Äď things like inline buffer formulation (IBF) and virus filtration ‚Äď must be completed with no deviation from accepted norms. For Asahi Kasei Bioprocess…
Antibody Internalization: Advanced Flow Cytometry and Live-Cell Analysis Give Rich Insights During Antibody Profiling
The natural characteristics of antibodies, such as high binding affinity, specificity to a wide variety of targets, and good stability, make them ideal therapeutic candidates for many diseases. Therapeutic antibodies, in particular monoclonal antibodies (mAbs), deliver promising therapeutic results in several different disease areas, such as autoimmunity, oncology, and chronic inflammation. Researchers‚Äô abilities to improve the breadth of antibodies have been aided by innovative technologies for antibody discovery, for instance, through humanization of mouse antibodies and phage display. However, advanced…
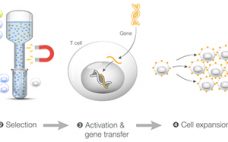
Integrated Generation and Characterization of CAR T Cells
Chimeric antigen receptor (CAR) T cell therapies have been approved by the FDA and other global public health agencies to treat B cell leukemias and have seen great clinical success. Autologous CAR T cell manufacturing involves isolating T cells from a patient, activating these cells, introducing an engineered CAR construct, and expanding the cells to a scale appropriate for therapeutic dosing. Patient samples from multiple sources result in inconsistent clinical outcomes and overall product quality. To ensure patient safety and…