Realizing the full potential of a novel injectable drug compound is no small task. In the months and years that lead from the exciting discovery phase to the rigorous demands of a commercial launch, unexpected scientific and technical challenges can slow development to a halt, often at key stages. A careful, systematic approach to identifying where and why these roadblocks can occur is fundamental to staying on course. Just as important is a robust, repeatable process design focused on retaining…
Manufacturing
Designing Quality into the Product: Early Developability Assessment in Biopharmaceutical Development
The ultimate objective in the development of any new therapeutic candidate is the validation of its mechanism of action and therapeutic efficacy in a clinical setting. Three important areas of product development critical to determining the success of a new drug candidate are: manufacturing, safety, and delivery of the product to patients. Early attrition observed during preclinical stages can often extend development timelines or require additional process optimization, therefore costing the developer more time and money. It is desirable to…
Control Over Your Microbial Identification Using the Gold-Standard Genotypic Method
Eliminate your outsourcing needs! Gain visibility and control. Learn how the next-generation, high-throughput MicroSEQ® system delivers right-first-time accuracy and give you complete control over microbial identification, by owning the system, the process and data analysis.
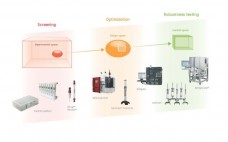
How to Achieve Greater Efficiency in Biopharmaceutical Process Development
Read about GE Healthcare Life Sciences’ support from efficient process development to production through products, consulting services as well as specific, individual collaborations. “Our aim is to help bioprocessing teams to map the optimal plan to transform an idea into results, with greater flexibility and confidence, reducing time and costs along the way. Our global team of bioprocessing experts will support you in the optimization and troubleshooting of existing unit operations or in the design of efficient and cost-effective processes…
Going Paperless from Lab to Plant
The drive to “go paperless” is a strategic initiative that offers demonstrable operational benefits in improving productivity, reducing cycle times and leveraging experimental and operational data along the entire research-development-manufacturing continuum. This technology brief reviews recent initiatives by science-based organizations to develop and deploy software that spans the lab-to-commercialization lifecycle using data standardization/harmonization technology and electronic lab notebooks (ELNs) to streamline technology transfer. Organizations adopting these proven informatics solutions can experience: Enhanced productivity Faster time to market Improved compliance More…
Supporting Advances in MAb Process Development and Manufacturing
Learn how GE Healthcare Life Sciences is supporting advances in MAb process development and manufacturing, and looking ahead, to Fabs and other antibody fragments that hold promise for new and more effective therapies.
The “early health” Vision
New approaches to vaccine production are needed. GE Healthcare Life Sciences contributes to the “early health” vision by supporting advances in vaccine development through innovative manufacturing solutions that help to shorten time-to-market, increase output, and assure quality.
Preclinical Immunogenicity Assessment
Protein therapeutics can potentially elicit immune responses when administered in humans. These antidrug antibodies could result in partial or complete loss of drug efficacy and other complications that have the potential to cause severe adverse effects in the patient. In this webcast, Philippe Stas, Head of Applied Protein Services at Lonza, discusses the benefits of preclinical immunogenicity assessment, including:
• Early-stage Risk Assessment
• Improved Quality and Safety of Drugs
• Reduced Attrition Rate in Drug Development Programs
View this webcast to learn more about managing drug-induced immune responses at the earliest possible stage to produce safer and more cost-effective protein therapeutics.
Quality, Speed and Flexibility by Design
Accelerating the path to new biomanufacturing capacity typically corresponds with increased costs and the risk of lower quality. Overcoming these compromises requires a holistic QbD-based rethinking of how biomanufacturing is approached. By rethinking biomanufacturing equipment, facility design and services, Xcellerex has achieved dramatic improvements in deployment time, while also reducing capital investment, operating costs, and improving overall quality and compliance. This webcast discusses the advantages of the FlexFactory® Biomanufacturing Platform, such as:
• Multi-product, simultaneous manufacturing
• Open platform for any bioprocessing technology/process control
• 70% reduction on time to build/validate to GMP ready (9mo.)
• And more.
Join Parrish Galliher, Founder and Chief Technology Officer at Xcellerex, as he provides an in-depth look at the technology behind Xcellerex’s FlexFactory Biomanufacturing Platform.
Industrial Purification with Convective Interaction Media™ Monoliths
Monoliths offer a unique set of characteristics that, when properly applied, can significantly improve the overall productivity of the manufacturing processes. In this educational webcast, Tony Brazzale of BIA Separations explores the technology behind the CIM™ Monolithic Columns and its many benefits, including:
• Increased facility capacity
• Accelerated ROI
• Improved productivity
• Reduced cost of labor and overall COGS
View this educational webcast to learn more about how CIM Monolithic Columns can improve your manufacturing process.