They’re far less exciting than the life-changing treatments they help create, but buffers play a critical role in upstream and downstream biomanufacturing processes. ANGUS takes you backstage with “Buffers in Biologics Manufacturing” – an in-depth article co-authored by Dr. Dave Green, Vice President of R&D, ANGUS, that explores the important variables to consider when selecting the right buffer (and buffer supplier). And, when it comes to biomanufacturing, we know a thing or two about buffers. Why? Because we invented them.…
Manufacturing
Single-Use Technology: The Next 5 Challenges to Conquer
Now that single-use technology plays a part in nearly all bio-production processes, what is next? Several challenges remain that are still serious enough to delay or even stop the use of single-use technology, which in turn can extend the time to market and increase the cost of lifesaving biopharmaceutical products. Of the existing difficulties, which five are the most critical? This paper considers five existing challenges in the implementation of single-use technology including scale, automation, product compatibility and compliance, speed…
Globalization of Single-Use and an Introduction to the Mobius® MyWay Portfolio
Single-use systems have enjoyed a meteoric rise in recent years and are fast becoming the go-to option for all but the biggest batches in the biopharma industry. But how do you get the most out of these exciting systems? Here, experts track the rapid evolution of single use systems, their impact on the industry and biomanufacturing, and how they will transform future manufacturing operations. Single-use is now becoming well established into an “age of optimization.” More than 90% of biopharma…
Single-Use Powder Containment for Biopharmaceutical Manufacturing
For many years, media and buffer ingredients used in powder form were transferred from stock containers using open scoops, weighed and mixed in buckets or open-top bags, and then carried in and dumped from those buckets or open-top bags directly into production vessels—based on the premise that sterility wasn’t required at that early stage of manufacturing. While much of this process was often carried out in a separate room from the production line to contain airborne contaminants, final transfer to…
BIOne – Single-Use Bioreactor System
Convert your existing benchtop glass bioreactor to a single-use bioreactor in seconds. Distek, Inc. has developed a benchtop scale single-use bioreactor (SUB) system for mammalian cell growth and recombinant protein production. The pre-sterilized BIOne system is engineered with a disposable headplate welded to a triple-layered liner that can be easily inserted into a non-sterile bioreactor glass vessel, converting it to a sterile, disposable SUB within a matter of seconds. Simply remove your existing headplate and place the preassembled and irradiated…
Implementing Global Best Practices and Technology Specifications for Single-Use Systems
As global markets become more and more important, so does global manufacturing. But when your company has several manufacturing sites across the world it can be difficult to streamline efforts, manage costs and share valuable information. Our recent white paper “Implementing global best practices and technology specifications for single-use systems” tackles the issues of multinational drug manufacturing and offers several best practices that can help you cut your time to market, lower probability of process troubleshooting during start-up and decrease…
Case Studies Using Mass Spectrometry to Characterize a Protein-Hapten Drug Substance
Clients often come to us with protein conjugation process needs that can require development of new and innovative methods to assure process validation for conformance manufacturing. Our staff utilizes state-of-the art technologies and instrumentation for a wide range of protein drug manufacturing challenges. These scientists provide the expertise and experience needed to develop accurate and highly reliable assay methods designed specifically to assure successful conformance manufacturing of their protein-hapten or protein-drug conjugate. This white paper illustrates just one example of…
The Standardization of Single-use Components for Bioprocessing
As single-use systems become more widely adopted, the focus in the bioprocessing industry is shifting from acceptance of the technology to standardization. While the standardization discussion encompasses many topics (including how products are tested, assembled, etc.), components are a critical area for improvement. With so many ways to apply single-use and hybrid bioprocessing systems, organizations must take action to standardize equipment in order to streamline operations and help reach the full potential of the technology. This paper discusses standardization in…
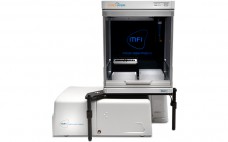
Solving Viscosity in Automated Particle Imaging
Micro-Flow Imaging™ (MFI) easily detects particle size and morphology on a wide range of particle contaminants. And when you add the Bot 1 Autosampler to your MFI 5000 Series system, you’ve got the automated go-to method of choice for particle analysis today. Together, this combination lets you quickly screen for any changes in levels of particle contaminants like protein aggregates and silicone oil in your biopharmaceutical formulation. Highly concentrated protein solutions can be a little trickier to analyze with other…
Your Reliable Contract Manufacturing Solution Partner in China
This whitepaper focuses on Boehringer Ingelheim’s development and manufacturing facility in China. As one of the world’s leading organizations in the area of biopharmaceutical contract manufacturing, Boehringer Ingelheim is the first multi-national pharmaceutical company that brings over 30 years of experience in biopharmaceutical contract manufacturing to China, to better serve patients and the biopharmaceutical industry – both in China and in the world. As a pioneer in biopharmaceuticals with more than 35 years of experience the company has brought more…