TOYOPEARL GigaCap DEAE-650M is an ideal anion exchange resin for the chromatographic purification of biotherapeutic products. Since the TOYOPEARL GigaCap DEAE-650M has such a high dynamic binding capacity, the need for very large columns and massive buffer consumption is minimized, particularly for proteins expressed at higher levels due to upstream improvements.
Downstream
A versatile high capacity, single-use chromatography tool with superior salt tolerance, process robustness and impurity removal
Strong anion exchange (Q) chromatography has become an industry standard in the polish purification steps of mAb production. It is a proven technology to remove DNA, viruses, endotoxins and acidic host cell proteins from process feed streams in flowthrough mode. As the industry pursues an increasing interest in downstream single-use technologies and flexible biomanufacturing due to advancements in cell culture technology and the emergence of cost-sensitive biosimilars, conventional purification technologies present limitations. Despite their high binding capacity, traditional resin-based chromatography…
Hydrophobic Interaction Chromatography: Effects of Mixed Electrolytes on Protein Separations
For HIC separations, parameters other than resin surface modifications can be employed to enhance performance. This application note addresses the electrolyte composition of the mobile phase as one parameter responsible for protein adsorption and desorption. The results presented illustrate the benefits regarding capacity and selectivity in HIC of often neglected salts and their mixtures.
Separation of Monoclonal Antibody (mAb) Monomer from its Half-body using Size Exclusion Chromatography
Recent research has shown an interest in mAb half-bodies as therapeutic vectors as they can be further targeted for conjugation, enzyme labeling, or antibody immobilization. The TSKgel SuperSW mAb HR is able to achieve high resolution between the mAb monomer, the mAb half-body, and fragments due to its unique pore-controlled technology optimized for mAb analysis, as well as its smaller 4 ÎĽm particle size.
Monoclonal Antibody Purification with a High Capacity Protein A Resin
“Protein A resins constitute a substantial cost in state-of-the-art mAb purification processes. Factors such as operating cycles, capacity, and mAb titer can have an impact on total costs associated with mAb purification. The purification of a monoclonal antibody from crude feed stock using TOYOPEARL® AF-rProtein A HC-650F, a high capacity protein A resin from Tosoh Bioscience, show that this particular high capacity protein A resin delivers highly pure antibodies at yields approaching 90%.”
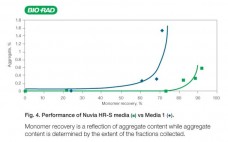
Introducing Nuvia HR-S Chromatography Media
Nuvia HR-S media is a new strong cation exchanger that has been optimized for particle size and chemistry that provides exceptional resolution and high recovery. Nuvia HR-S media demonstrates fast mass transfer kinetics, excellent flow characteristics, and robust chemical stability against common caustic cleaning protocols. Its excellent scalability gives process developers the confidence that results obtained on the bench will be reproducible for large-scale downstream manufacturing. Nuvia HR-S media is the preferred solution for intermediate and final polish applications where…
TOYOPEARL AF-rProtein A HC-650F Host Cell Protein Removal
The following paper compares the host cell protein (HCP) removal capabilities of TOYOPEARL AF-rProtein A HC-650F, TOYOPEARL AF-rProtein A-650F, and another commercially available high capacity protein A resin.
A Platform Approach for the Purification of Domain Antibodies (Dabs)
A three-step purification process for a Dab successfully developed and verified. The step yield ranged between 86% and 99%, giving a total process yield of approximately 81%. The ECP in the start sample was more than 200 000 ppm whereas the final sample contained only 5.5 ppm. The endotoxin content in the feed was approximately 2 million endotoxin units/mg of protein and the final sample was below the limit of quantification. Protein L leakage was undetectable in all samples. This…
MFI for Particle Characterization of Biopharmaceuticals Today
Micro-flow imaging (MFI) is a sensitive, simple and automated method for the analysis of sub-visible particles and translucent protein aggregates that provides particle size, count and morphology. Because it quantifies particle shape, this technology can discriminate groups of particles from each other, and evaluate how these groups change over time. In this article, we provide an overview of how biopharma is using MFI to characterize and monitor their protein formulations in new and emerging applications.
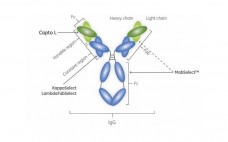
POROS® CaptureSelect™ Affinity Columns for Rapid, Small-Scale Purification and Sample Preparation of Recombinant Proteins
This application note describes a model system in which Enbrel® protein, a fusion protein consisting of TNF receptor and IgG1 Fc, is purified from a dilute sample matrix for further analytical characterization. The resulting purified protein was subjected to N-linked glycan structural analysis by mass spectrometry (MS) and its aggregation state was assessed by analytical ultracentrifugation (AUC). This data set serves as a model to demonstrate the capability of the POROS® CaptureSelect™ product line for the affinity purification of biomolecules…