This eBook presents various techniques used to measure the stability of antibody-based biotherapeutics. The authors address the ins-and-outs of monoclonal antibody (MAb) formulation, cover Investigational New Drug (IND) and New Drug Application (NDA) filing timelines, and explain how storage conditions affect MAb stability. Monoclonal antibodies have been used in therapeutics for more than 30 years. Efforts to further develop and optimize better MAb-based biotherapeutics are increasing as they have become increasingly popular for the treatment and prevention of many diseases…
Downstream
Future Proof Your HCP Strategy
Future proof your HCP strategy. Four questions to help you decide if your HCP analysis data is ready for next phase approval. Host cell proteins (HCPs) are a primary source of impurities in biologics development. Scientists must detect and remove HCPs to ensure patient safety and meet regulatory guidelines. If previously undetected HCPs are found in later phases of clinical trials, scientists will need to redesign the drug substance purification strategy – adding financial and time costs that can create…
Do Not Let HCPs Delay Your Biologics Route to Market
The stakes are high in biologics development, especially in host cell protein (HCP) analysis. Regulatory bodies have rules and suggested strategies for HCP analysis and reporting, and it is likely that removing HCPs is a key concern in your process development. Ultimately, the accuracy of monitoring and success of purifying your biologic of these HCPs can make or break your product. Get it wrong and it could delay your development cycle. In this blog, we outline strategies to optimize your…
Ensuring Everything is A-OK at AKBA
Accurately and reliably producing pharmaceuticals and biotherapeutics is one of the most important – and challenging – tasks in the world of manufacturing. Any misstep in the production process will lead to the creation of a substandard end product that can do substantial harm to the user. That’s why the processes used in these production chains – things like inline buffer formulation (IBF) and virus filtration – must be completed with no deviation from accepted norms. For Asahi Kasei Bioprocess…
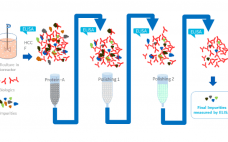
Confidence in Host Cell Protein (HCP) Coverage Assays with Differential Gel Approaches
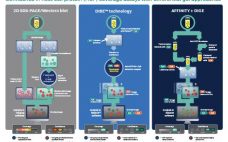
Enzyme-linked immunosorbent assays (ELISAs) are critical to detecting and removing host cell proteins (HCPs), a primary source of impurities in biologics development. Scientists must validate HCP ELISAs to ensure patient safety and meet regulatory guidelines – but different coverage assays come with different challenges, limitations, and benefits. The US Pharmacopeia recommends 2D differential gel electrophoresis (DIGE) combined with Western blot or immunoaffinity approaches, and labs might not be able to gain the full benefits of DIGE without significantly altering current…
Why, Why, Why… ELISA? A Look at the Benchmark HCP Assay
Host cell proteins (HCPs) are a primary source of impurity in biologics manufacturing. When present in drug formulations, HCPs can reduce efficacy, introduce toxicity, and increase risk of long-term immunogenicity. Understanding HCP profiles and integrating effective removal strategies are critical when developing a new biological drug, both for ensuring patient safety and fulfilling regulatory guidelines. HCP populations can be complex and structurally diverse, and most changes in upstream culture conditions affect HCP concentrations and control strategies. Accurate and reliable HCP…
Cyclic Cell Harvest with CONTIBAC® SU Filters
As the Biotech industry is moving towards single-use components, larger batch volumes and higher cell concentrations, conventional cell harvest technologies reach their limit. Depth filters, for instance, can only cope with the increasing demands by stacking more filter elements and therefore increasing the footprint and their economic burden. Hence, innovative solutions are needed to keep pushing the boundaries of the biologics production. The CONTIBAC® SU filter of DrM excels where existing technologies crumble. Unlike in any competing technology, the filtration…
Why Characterizing Protein Stability Matters For Drug Development
Characterizing a protein’s stability provides key insights into the expression, signaling, and regulatory roles of a molecule. This is necessary for numerous applications from understanding the molecular basis for certain diseases to ensuring more effective drug development. Ultimately, the stability of a protein is closely tied to its functional activity. Whether you’re just starting to learn about characterizing protein stability or you’re looking for a refresher, this guide has some helpful tips to answer why it’s important, what technologies are…
BioContinuum™ Buffer Delivery Platform
Streamline your buffer preparation and management through an integrated combination of an automated buffer dilution system, high-quality buffer concentrates, single-use assemblies, and a tailored service package.
New Features for Single-Use Pumps in Biopharmaceutical Manufacturing
Everybody has heard the axiom “time is money,” which highlights the belief that time is a valuable resource; therefore, it is better to do things as quickly as possible in order to make more money. This concept perfectly describes the current atmosphere within the biopharmaceutical industry, where drug manufacturers are struggling to overcome speed-to-market challenges in order to reap the financial benefits of an optimized patent window. In order to bring their products to market, most biopharmaceutical-manufacturing systems employ a…