The rapidly growing interest in gene therapy has led to the need for more cost-effective and scalable viral vector manufacturing platforms to deliver these therapies. Adeno-associated virus (AAV) has become the vector of choice as it stands out for its safety profile since infection with the vector is not pathogenic. Also, AAV cannot replicate on its own and is not directly integrated into the host genome. Realizing the full potential of viral vector-based therapies requires a successful manufacturing platform for…
Cell Therapies
Selecting the Best Transfection Method
When to Use Transfection Reagents, Viral Transduction or Electroporation No single delivery method is ideal for all situations, but researchers may routinely employ a suboptimal approach for the sake of familiarity or to avoid any start-up costs associated with new methods. In this white paper, we will describe three methods (chemical, electroporation and viral transduction) and highlight the Mirus Bio TransITĀ® and VirusGENĀ® transfection reagents and IngenioĀ® EZporatorĀ® Electroporation System, which are both easy to use and cost-effective. Additionally, we…
Transfection Reagents for Cell and Gene Therapy
Polysciences offers high-quality, R&D and cGMP grades of transfection reagents for the smooth transition from process development through clinical trials and commercial manufacturing of viral vectors/protein. Polysciences R&D grade PEI MAX has been used by academic and industry customers for over a decade and is one of the most cited transfected reagents. A few years ago Polysciences launched an easy to use liquid version of PEI MAX which is called Transporter 5 focusing on increased customer convenience. Both PEI MAX…
Manufacturing Pluripotent Cell Therapeutics
Here we review strategies for gaining Food and Drug Administration (FDA) approval of allogeneic, pluripotent cell therapies. The crux of the discussion is that when developing a cell therapeutic, it is critical to look as much as a decade ahead to when FDA approval will be sought to commercialize the product through a biologics license application (BLA). While this discussion focuses on FDA approval of cell therapies, it is important to acknowledge the vast number of cell therapy clinical efforts…
Scalability of Lentiviral Production with the CTS LV-MAX Lentiviral Production System in Bioreactors
The Gibco™ CTS™ LV-MAX™ Lentiviral Production System provides a scalable and high-yield lentiviral vector (LV) production platform. It is based on a high-density suspension culture of HEK293Fāderived Viral Production Cells that have been optimized for viral production in chemically defined LV-MAX Production Medium. Scalable LV production of greater than 1 x 10āø TU/mL LV (unconcentrated) can be achieved using our proprietary lipid nanoparticle transfection reagent in combination with an LV-specific enhancer and production supplement. All components work synergistically to help…
Automated PBMC Isolation and T Cell Wash and Concentration by the CTS Rotea System
Successful processing and manufacturing in cell and gene therapy workflows are essential to the efficacy of the product. Autologous cell and gene therapy workflows involve isolating cells from an individual, engineering the cells, expanding and concentrating them, and infusing them back into the patient. Certain steps in these workflows could benefit from optimized automation to decrease hands-on time and the cost of the cell manufacturing process. In this application note, we present the Gibco™ Cell Therapy Systems™ (CTS™) Rotea™ Counterflow…
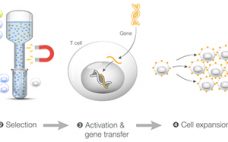
Integrated Generation and Characterization of CAR T Cells
Chimeric antigen receptor (CAR) T cell therapies have been approved by the FDA and other global public health agencies to treat B cell leukemias and have seen great clinical success. Autologous CAR T cell manufacturing involves isolating T cells from a patient, activating these cells, introducing an engineered CAR construct, and expanding the cells to a scale appropriate for therapeutic dosing. Patient samples from multiple sources result in inconsistent clinical outcomes and overall product quality. To ensure patient safety and…
Effectively Securing Cell and Gene Therapies with Closed Systems
With their innovative new treatments, cell and gene therapies (CGT) are growing rapidly. But their traditional production processes donāt allow the supply of these therapies to keep up with demand. Historically, these therapies are produced for small patient populations in clinical trials using laboratory scale equipment and utilizing manual, open processes completed under laminar hoods. But manufacturers looking for more efficiency and flexibility are turning to new solutions. One key area of interest is the implementation of a closed system…
Ten important lessons the cell and gene therapy industry can take from the bioprocessing industry
Itās time to tackle the challenges of sustainable and cost-effective commercial manufacturing regarding cell and gene therapy. Cell and gene therapies have many of the same manufacturing needs as biopharmaceuticals. As a result, industry experts expect single-use technologies used in biopharmaceutical clinical trials and commercial production to play a larger role in the future development and production of cell and gene therapy. Single-use systems are already incorporated in the development of cell and gene therapies today. However, many of those…
Directed Differentiation of cGMP Compliant Human Induced Pluripotent Stem Cells Into Clinically Relevant Specialized Cells From All Three Germ Layers
The generation of human induced pluripotent stem cells (iPSCs) via reprogramming technology represents a major breakthrough in personalized medicine and the treatment of degenerative diseases. This is mainly because the iPSCs can be expanded in culture and then differentiated into specialized cell types that can be used for clinical applications. Patient-derived iPSCs can be used to model human genetic diseases, produce clinically relevant differentiated cells that display disease pathogenesis, or generate specialized cells through directed differentiation process for autologous cell…