Fujifilm Irvine Scientific says a third facility at its Tilburg site will help meet future demand for cell culture media from biomanufacturers. Plans have been laid for a 250,000 square-foot building at Fujifilm‚Äôs site in Tilburg, The Netherlands to produce a range of media for the biomanufacturing space. When operations begin ‚Äď expected late 2021 ‚Äď the plant will increase Fujifilm Irvine Scientific‚Äôs production capacity by 320,000 Kg per year for dry powder, and 470,000 L per year for liquids.…

Upstream & Downstream Processing
ambr alert: Sartorius vessel specialized for cell & gene therapies
Sartorius has launched an ambr 250 vessel for cell and gene therapy applications it says taps into industry‚Äôs transition from adherent to suspension cell culture formats. Sartorius Stedim Biotech (SSB) has added to its range of ambr 250 modular benchtop bioreactor systems with a single-use vessel designed to accelerate the process development of cell and gene therapy applications, before scaling-up into single-use bioreactors and bags. Existing ambr mammalian vessels are often used for process development of monoclonal antibodies (MAbs) in…
MilliporeSigma plans to ramp up filtration manufacture in NH
MilliporeSigma intends to add an additional 38,500 square feet of manufacturing space at its site in New Hampshire to support demand for filtration devices. The bioprocessing business unit of Germany‚Äôs Merck KGaA, MilliporeSigma, has revealed it is planning to expand a site in Jaffrey, New Hampshire that makes filtration equipment for the life sciences industry. ‚ÄúTo meet the future demands of the bioprocessing market, we are in the approval process to expand the facility with additional cleanroom and lab space…
Middle class: Lonza on the flexibility of midscale biomanufacturing
With construction of a 6,000 L scale hybrid plant underway, CDMO Lonza says there is high demand among its customers for midscale biomanufacturing. In this industry, companies and commentators like talking about extremes: either small-scale single-use or large-scale stainless steel. But speaking at the BPI Theater at BIO in Philadelphia earlier this month, St√©phane Varray, commercial development for midscale at Lonza Pharma and Biotech said midscale options provide firms with flexibility across numerous modalities. ‚ÄúWhen we look at midscale we’re…
Fujifilm tackles buffer bottleneck at $10m continuous processing plant
Fujifilm Diosynth Biotechnologies says it can overcome the burden of large buffer volumes associated with continuous purification as part of a new UK processing facility. The facility in Billingham, UK will include a 500 L single-use perfusion bioreactor and 7 downstream processing units and is expected to begin offering process development later this year. The plant represents a $10 million (‚ā¨8.9 million) investment by the contract development and manufacturing organization (CDMO) and is the culmination of three years of in-house…
Inside Jefferson: $7m bioprocess training institute opens in PA
The Jefferson Institute for Bioprocessing (JIB) has opened its doors in Pennsylvania. Bioprocess Insider visited to find out how the site, supported by Ireland‚Äôs NIBRT and GE Healthcare, will aid the bioprocess industry. In June 2018, plans were laid down for a site in Pennsylvania for an education and training institute to support the staffing gaps in the bioprocessing space. A year on, the Jefferson Institute for Bioprocessing (JIB) has opened, with help from a $7 million (‚ā¨6.2 million) investment…
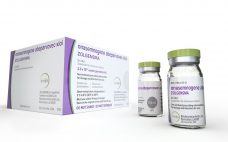
Zolgensma: We all know the price, but how is it made?
Making Zolgensma, the new ‚Äúworld‚Äôs most expensive drug,‚ÄĚ is a month-long process that depends on vector tech licensed from Regenxbio. Zolgensma is a gene therapy. It is designed to halt progressive spinal muscular atrophy (SMA) ‚Äď motor neurone loss and muscle wasting ‚Äď in people who have a defective version of the gene SMN1. The drug addresses the underlying causes of SMA according to Novartis spokesman Eric Althoff. He told us, ‚ÄúZolgensma provides a functional copy of the human SMN…
Pluristem looks to cell cultured cannabinoids
Pluristem Therapeutics has filed a provisional patent application to use its 3D cell culturing technology to manufacture cannabinoid-producing cells. Pluristem has several candidates in its pipeline based on placenta-derived, mesenchymal-like adherent stromal cells, has inked a recent study agreement with NASA, and is working with Thermo Fisher to address the lack of technologies available to cell therapy manufacturers for the cultivation and processing of allogeneic products. Now the Israeli cell therapy firm is looking to use its cell culture platform…
UNSW looks to ‚Äėsubmarine-like‚Äô microparticles for drug delivery
Micro-motors developed by the University of New South Wales (UNSW) could respond to variations in biological environments to navigate and attack tumor cells. Findings from the research undertaken at Sydney, Australia-based UNSW have been published in Material Today, under the title ‚ÄėBiocatalytic self-propelled submarine-like metal-organic framework microparticles with pH-triggered buoyancy control for directional vertical motion.‚Äô The paper shows evidence of ‚Äúself-propelled submarine-like microparticles‚ÄĚ that carry drugs and use changes in the biological environments and pH to navigate to specific areas…
Costs and quality will shape cell and gene therapy tech development
Cell and gene therapies as well as wider industry desire for cost efficiency will shape bioprocessing tech innovation, say experts. Biopharma interest in cell and gene therapies is increasing. Products like Kymriah, Luxturna and Yescarta are the tip of an R&D iceberg according to PhRMA, which says around 300 such treatments are in development. The trend has been well received, with various groups suggesting it will yield treatments for untreatable conditions (see here, here and here, for examples). Investors have…